Figure 1.
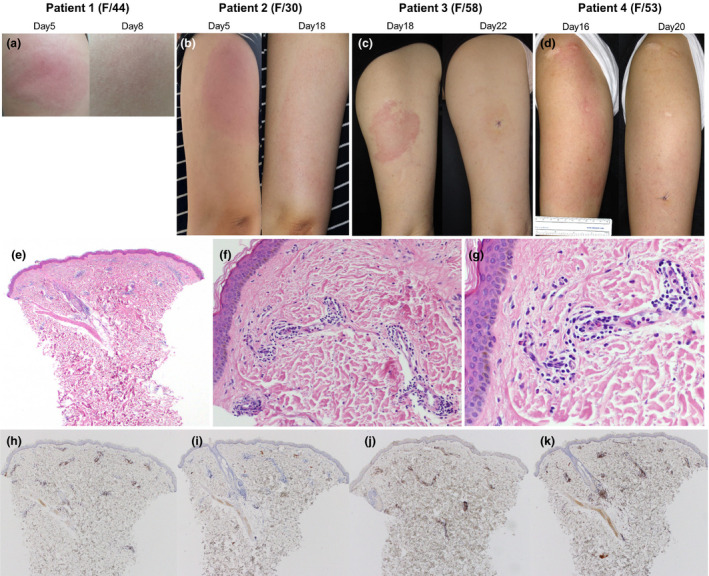
Clinical photographs and the representative histopathologic findings of delayed cutaneous reactions to the ChAdOx1 nCoV‐19 vaccine. (a) Patient 1 presented considerable induration with pain and tenderness on Day 5. After 3 days of oral prednisolone 30 mg, the skin lesion resolved. (b) Patient 2 started a painful erythematous swelling on Day 5. Without treatment, the skin lesion became larger (15 × 8 cm), but pain decreased, and pruritus occurred on Day 18. The skin lesion gradually improved after 3 days of oral antihistamine and topical corticosteroid. (c) Patient 3 presented with erythematous tender plaque on day 17. There was no immediate skin reaction after vaccination. On Day 18, the patient underwent skin biopsy and was prescribed oral antihistamine and topical corticosteroid. Four days later (on Day 22), the skin lesion was much improved. (d) Patient 4 suffered from 18 × 10 cm sized itchy erythematous swollen plaque on Day 16. There was no immediate cutaneous symptom after vaccination and no concurrent systemic symptom. (e–g) Skin biopsy specimen from patient 4 shows superficial perivascular and perifollicular lymphocytic infiltration and some eosinophils. Neutrophils are present inside dilated small vessels. Immunohistochemistry reveals mainly CD3+ T cells with sparse exocytosis (h) and few CD20+ B cells (i). There is a mixed population of CD4+ (j) and CD8+ (k) cells, but CD4+ is predominant. These findings are consistent with a delayed hypersensitivity reaction (e: H&E ×40, f: H&E ×200, g: H&E ×400, h: CD3 ×40, i: CD20 ×40, j: CD4 ×40, k: CD8 ×40).
