Abstract
General anesthesia and surgery are associated with an increase in neural injury biomarkers. Elevations of these neural injury biomarkers in the perioperative period are associated with postoperative delirium. Xenon has been shown to be protective against a range of neurological insults in animal models. It remains to be seen if xenon anesthesia is neuroprotective in the perioperative setting in humans. Twenty-four participants scheduled for lithotripsy were randomized to receive either xenon or sevoflurane general anesthesia. There was no statistically significant difference in the concentrations of postoperative neural injury biomarkers between the xenon and sevoflurane group. Following the procedure there was a significant increase in the concentration from baseline of all three biomarkers at 1 hour post-induction with a return to baseline at 5 hours. General anesthesia for lithotripsy was associated with a significant increase at 1 hour post-induction in the neural injury biomarkers total tau, neurofilament light and tau phosphorylated at threonine 181, a marker of tau phosphorylation. The protocol was approved by the St. Vincent’s Hospital Melbourne Ethics Committee (approval No. HREC/18/SVHM/221) on July 20, 2018 and was registered with the Australia New Zealand Clinical Trials Registry (registration No. ACTRN12618000916246) on May 31, 2018.
Keywords: biomarkers, inflammation, lithotripsy, neurocognitive disorders, neurofilament; neuroprotection, postoperative delirium, tau, xenon
INTRODUCTION
Postoperative neurocognitive disorders are common and related to significant morbidity and mortality.1 The role of general anesthesia in the development of postoperative cognitive dysfunction remains controversial. Previous studies have identified a number of potential mechanisms in animal models including neuroapoptosis,2,3 enhanced amyloid production,4,5 and tau phosphorylation6 by which general anesthetic agents may induce cognitive dysfunction. Despite this, there is no evidence that the choice of anesthetic technique, regional, intravenous, or inhalational general anesthesia alters the trajectory of cognitive impairment following surgery.7,8
The brain biomarkers, total tau (T-tau) protein and neurofilament light (NFL), have been identified as tools to monitor neural injury in neurodegenerative conditions,9,10 traumatic brain injury11,12 and following cardiac arrest.13 Elevations in these markers of neural injury have been identified following anesthesia and surgery14 and greater elevations of NFL15 and T-tau16 postoperatively are associated with postoperative delirium (POD). A recent study has also utilized these markers in a patient cohort that received general anesthesia without surgery in an attempt to distinguish the effects of anesthesia and surgery on neural injury.17
A novel blood biomarker of Alzheimer’s disease (AD) pathophysiology is tau phosphorylated at threonine 181 (P-tau181).9 P-tau 181 can differentiate mild cognitive impairment from AD and high levels are associated with subsequent development of AD in cognitively normal adults.18 Exposure to general anesthetic agents have been associated with elevated P-tau181 in a number of animal studies.19,20,21 Phosphorylated forms of tau protein measured in the blood are also thought to more accurately reflect the abundance of tau in the central nervous system than T-tau.22 Whilst P-tau181 is not a direct marker of neural injury,23 a perioperative increase in P-tau181 could indicate an alternative mechanism by which general anesthesia can induce cognitive dysfunction.
In contrast to the other general anesthetic agents, xenon has been identified in animal models to be less neurotoxic2,24 and neuroprotective in a number of models of neural injury.25 A recent review identified potential benefits of xenon anesthesia such as hemodynamic stability and cytoprotection as being particularly beneficial in the elderly surgical population.26
There is conflicting evidence of both neurotoxic and neuroprotective effects of sevoflurane on the brain in animal models. In animal models without underlying pathology, sevoflurane is associated with dose-dependent cognitive decline.27 However, in hypoxemic brain injured animals it offers neuroprotection.28 A recent review of experimental studies concluded that although sevoflurane likely offers neuroprotection against brain injury, it also seems to be neurotoxic in both neonatal and aged animal models.29
Despite evidence of neuroprotection in animal studies, the ability of xenon as the anesthetic agent to provide improved cognitive outcomes in the perioperative period remains uncertain. Whilst general anesthesia with xenon trended toward a reduction in POD in elderly subjects requiring surgery following femoral neck fractures when compared to sevoflurane, this did not achieve statistical significance.30 A pilot study in off-pump cardiac surgery suggested that xenon anesthesia reduced the incidence of POD compared to conventional inhalational anesthesia31; however a larger study from the same group investigating on-pump cardiac surgery showed no difference in POD incidence for xenon anesthesia when compared to the inhalational anesthesia group.32
The neuroprotective potential of xenon has also been studied in the intensive care setting. Inhaled xenon in conjunction with hypothermia following out of hospital cardiac arrest was associated with improved brain imaging outcomes in a pilot study when compared to hypothermia alone. A larger clinical trial of the utility of xenon in this setting is currently underway.33
The primary hypothesis of this study was that general anesthesia with xenon for extracorporeal shock wave lithotripsy would result in reduced neural injury. The secondary hypothesis was that inhalational general anesthesia for extracorporeal shock wave lithotripsy, with either agent, is associated with neural injury.
The primary outcome of the study was a comparison of the concentration of postoperative neural injury biomarkers between xenon and sevoflurane anesthetic groups. Secondary outcomes included the change in biomarkers from baseline at 1-, 5- and 24-hours post-induction for all participants and comparison of the Montreal Cognitive Assessment (MoCA) before and following anesthesia. A modified Brice questionnaire was performed, and bispectral index (Medtronic, Minneapolis, MN, USA) values were also recorded.
SUBJECTS AND METHODS
Written informed consent for this randomized single-blind control trial was obtained from all participants. The protocol was approved by the St. Vincent’s Hospital Melbourne Ethics Committee (approval No. HREC/18/SVHM/221) on July 20, 2018 and was conducted at a single centre, St. Vincent’s Hospital, Melbourne. The study protocol was registered with the Australia New Zealand Clinical Trials Registry (registration No. ACTRN12618000916246) on May 31, 2018. An analysis of electroencephalograph recordings from the same participants has been reported elsewhere.34
Study population
The inclusion criteria were American Society of Anesthesiologists (ASA) class I and II patients aged 50 years and over scheduled for extra-corporeal shockwave lithotripsy. All patients listed for this procedure on the St. Vincent’s Hospital Patient Administration System during the study period were screened for eligibility according to age and location. Those deemed eligible were contacted by a member of the research team by telephone for further screening and a patient information flyer was sent to interested patients. Formal written consent was obtained on the day of admission. This surgical cohort was selected as a sample of relatively healthy adult patients receiving a highly standardized and relatively minor procedure that is usually performed under general anesthesia in our institution. Exclusion criteria were a diagnosed neurocognitive condition, high risk of postoperative nausea and vomiting (postoperative nausea and vomiting incidence following general anesthesia with xenon has been shown to be greater than with volatile agents),35 contraindication to the use of a laryngeal mask airway, severe respiratory disease (unable to tolerate inspired oxygen concentration of 35%) or any contraindication to anesthesia with a volatile agent. Participants were also required to return to the hospital the following day for various assessments and therefore living or staying in proximity to the hospital was also a consideration.
Randomization to either the xenon or sevoflurane group was achieved by computer generated random permuted blocks of four (Stata/IC 14.2, Stata Corp., College Station, TX, USA) provided in sequential opaque sealed envelopes. Although the anesthesiologist was unable to be blinded, the participants and research staff collecting data were unaware of treatment allocation. All data analysis was performed by research staff blinded to the treatment allocation.
Anesthetic protocol
All participants received preoxygenation via a circle circuit for 5 minutes prior to induction. A remifentanil (Symbion, Melbourne, Australia) infusion at a dose of 0.1 µg/kg per minute was commenced simultaneously with preoxygenation. Anesthesia was induced with a bolus of propofol 2 mg/kg (Symbion) and an appropriately sized laryngeal mask airway was placed. Additional boluses of propofol could be given at the discretion of the anesthesiologist to facilitate airway placement.
Anesthesia was maintained with a target end-tidal concentration of 60% xenon (minimum alveolar concentration of xenon is estimated as 63%)36 in the xenon group and a target end-tidal concentration of 0.9 of the age adjusted minimum alveolar concentration of sevoflurane (Symbion) in the sevoflurane group. The inspired oxygen concentration was 35% in both groups. All participants’ lungs were ventilated with a tidal volume of 6 mL/kg and the respiratory rate titrated to achieve normocapnea, as measured by end-tidal carbon dioxide monitoring. No participants received neuromuscular blocking agents.
In the event of hemodynamic changes suggestive of inadequate or excessive anesthesia (change in mean arterial pressure or heart rate > 20% from baseline) the remifentanil infusion rate could be titrated accordingly. Titration of the remifentanil rate and additional propofol boluses could also be administered at the discretion of the anesthesiologist in response to clinical evidence of inadequate anesthetic depth (e.g., movement, coughing or laryngospasm). Participants received paracetamol 1 g intravenous intraoperatively for postoperative analgesia and droperidol 625 µg and ondansetron 4 mg at the conclusion of the procedure for anti-emesis. Following recovery of consciousness and removal of the laryngeal mask airway participants were transferred to the post-anesthetic care unit for routine postoperative monitoring and care.
Medical grade xenon was provided by Coregas Australia (Coregas Pty Ltd., Thomastown, Australia). Xenon anesthesia was administered using the Akzent X Color (Stephan GmBH, Gackenbach, Germany) anesthetic machine. Xenon gas was delivered via a semi-closed circuit to minimize wastage. Sevoflurane anesthesia was administered using the Draeger Primus anesthesia machine (Draeger Australia Pty. Ltd., Melbourne, Australia) in a low-flow circle circuit.
Blood sample collection and analysis
Venous blood samples were obtained from participants at four time points, immediately prior to induction of anesthesia, 1, 5, and 24 hours post-induction. A sample at 72 hours post-induction was initially planned but it proved a significant impediment to recruitment, requiring participants to return to the hospital on two separate occasions following their day surgery procedure. As a result, this time point was not included in the study. At each time point, excepting the 24-hour time point, a 4 mL blood sample was taken from the intravenous catheter placed for the anesthetic induction. The 24-hour sample was taken by venipuncture performed by a member of the research team. All samples were collected in ethylenediaminetetraacetic acid tubes, stored on ice, and centrifuged within 1 hour at 3345 × g for 10 minutes. Plasma was then aliquoted into 500 µL tubes and rapidly frozen and stored at –80°C.
At the conclusion of the study, plasma samples were frozen and transported to the Clinical Neurochemistry Laboratory at the Sahlgrenska University Hospital, Mölndal, Sweden. T-tau and NFL concentrations were measured using commercial Simoa kits, NF-light and Tau 2.0 (Quanterix, Billerica, MA, USA) on an HD-X instrument. Plasma P-tau181 was measured using an in-house Simoa assay developed at the Sahlgrenska University Hospital, as described previously in detail.9 Analyses were performed on a Simoa HD-1 instrument (Quanterix, Billerica, MA, USA).
The values for all three biomarkers are reported in pg/mL. The measurements were performed in one round of experiments using one batch of reagents by board-certified laboratory technicians who were blinded to clinical data. Samples from the same individual were analyzed side-by-side on the same plate to minimize variation. Intra-assay coefficients of variation, monitored using high and low quality control samples at the beginning and end of each plate, were 1.4–8.8%, with the exception of the low P-tau internal control sample that had a coefficients of variation of 17%.
Cognitive assessment and Brice questionnaire
All participants underwent a brief 10-minute screening test for mild cognitive impairment and AD, the MoCA.37 The MoCA was administered by a blinded member of the research team and it was administered at three timepoints: admission on the morning prior to anesthesia and surgery, 5 and 24 hours post-induction of anesthesia.
Participants planned as day case surgery were discharged 5 hours post-procedure. Participants requiring overnight admission were all discharged the following day. Following discharge participants were contacted by telephone by a member of the research staff and a modified Brice questionnaire38 was administered to identify any episode of awareness in the form of recall.
Sample size calculation
Previous work published by members of our research team identified that the mean peak percentage increase, from baseline, in T-tau following anesthesia and surgery was 257%.14 In a preliminary analysis of postoperative outcomes in the same cohort, the participants who did not develop POD during admission were found to have postoperative tau levels around 30% lower than those who did (unpublished data, mean T-tau 4.2 pg/mL, 95% confidence interval 2.4–6 pg/mL, versus 6.4 pg/mL, 95% confidence interval 4.9–7.9 pg/mL). Therefore, we based our power calculation on detecting a 30% reduction in the mean peak percentage increase in T-tau for participants in the xenon group when compared to the sevoflurane group. Assuming a power of 80% and an alpha of 0.05, 18 participants in total (9 in each group) would be required to detect a reduction of 30% in the magnitude of rise in T-tau as per a mean comparison performed in Stata (Stata/IC 14.2, Stata Corp., College Station, TX, USA). Allowing for a 25% loss to follow-up we planned to recruit 24 participants.
Statistical analysis
Normally distributed data was compared using Student’s t-test and are reported as mean and standard deviation. Biomarker data was log transformed and a mixed effects model was fitted to compare the biomarker concentration between groups at each of the three post-operative time points. The model was fitted with three co-variates: anesthetic group, postoperative sampling time point and baseline biomarker value (Model 1). A second mixed effects model was fitted to compare the biomarker concentration of all participants at each postoperative time point to the baseline values (Model 2). This model was fitted with two variables: anesthetic group and sampling time point. Results of both models are reported as exponentiated coefficients and 95% confidence intervals. The exponentiated coefficient describes the estimated ratio of biomarker concentration in the sevoflurane versus the xenon group (Model 1) and the estimated ratio of biomarker concentration at each time point versus baseline values (Model 2). All statistical analysis was performed in Stata (Stata/IC 16.0).
RESULTS
A total of 24 participants were recruited for the study between October 2018 and September 2019, 12 in each group (Figure 1). One participant allocated to the xenon group received sevoflurane due to a malfunction of the xenon delivery apparatus. The malfunction was identified during testing prior to the participant entering the operating theater. One participant randomized to the sevoflurane group withdrew consent prior to administration of the anesthetic. The results below are based on a per-protocol analysis. Participants in the two groups were comparable with respect to age, height, weight and ASA status (Table 1).
Figure 1.
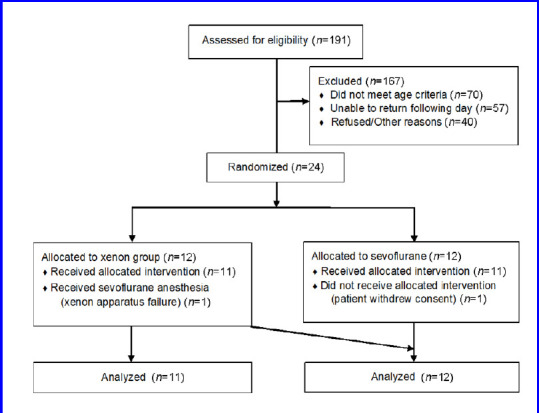
CONsolidated Standards Of Reporting Trials (CONSORT) diagram.
Table 1.
Patient and anesthesia characteristics for xenon and sevoflurane groups
| Xenon (n=11) | Sevoflurane (n=12) | P-value | |
|---|---|---|---|
| Age (yr) | 60 (56–64) | 62 (56–68) | 0.56 |
| Height (cm) | 170.0±7.0 | 170.0±9.6 | 0.92 |
| Weight (kg) | 77.0±16.0 | 78.0±11.8 | 0.85 |
| Male | 6 (55) | 10 (83) | 0.17 |
| American Society of Anesthesiologists II | 2 (18) | 1 (8) | 0.59 |
| Baseline mean arterial pressure (mmHg) | 98.0±13.4 | 100.0±6.0 | 0.65 |
| Maintenance mean arterial pressure (mmHg) | 80.1±7.5 | 73.3±6.1 | 0.03 |
| Total propofol (mg) | 257.3±74.6 | 215.8±52.5 | 0.14 |
| Total remifentanil (μg) | 513.9±318.8 | 479.4±286.3 | 0.79 |
| Total ephedrine (mg) | 10.9±8.0 | 24.5±17.2 | 0.03 |
| Duration anaesthetic (min) | 44.5±15.8 | 44.6±5.3 | 0.98 |
| End tidal xenon (%) | 56.6±2.6 | NA | |
| End tidal sevoflurane (%) | NA | 1.72±0.2 | |
| Mean bispectral index value | 37.8±4.9 | 37.2±5.4 | 0.84 |
| Recovery (min) | 5.4±2.0 | 11.0±5.3 | < 0.01 |
Note: Data for age are presented as mean (range). Data for height, weight, mean arterial pressure, total propofol dose, total remifentanil dose, total ephedrine dose, duration of anaesthetic, end-tidal xenon and sevoflurane, mean bispectral index value and recovery time are presented as mean ± SD. Male and American Society of Anesthesiologists classification I are shown as number (proportion). NA: Not applicable.
Patient and anesthesia characteristics
The duration of anesthetic, from induction until inhalational delivery ceased, total propofol and remifentanil dose and mean bispectral index value during maintenance were comparable between groups (Table 1). The mean time in minutes from cessation of inhalational agent to opening eyes to voice was significantly shorter in the xenon group when compared to the sevoflurane group (P < 0.01).
Comparison of biomarker concentration between groups (Model 1)
There was no statistically significant difference between groups in the concentration of T-tau protein, P-tau181 or NFL at any of the three postoperative time points, adjusting for baseline (Figure 2 and Table 2).
Figure 2.
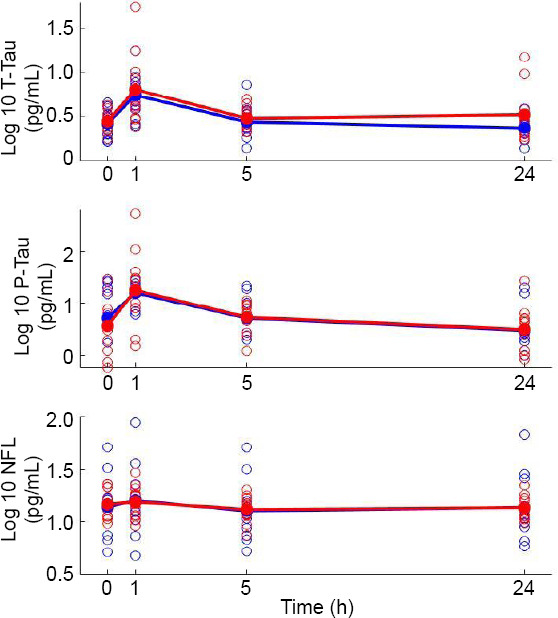
Change in biomarkers over time.
Note: Log-transformed values for each participant at each time point are represented by unfilled circles, blue indicates xenon participant, and red indicates sevoflurane participant. Filled circles and line represent log - transformed mean value of each anesthetic group, blue indicates xenon participart, and red indicates sevoflurane participart.
Table 2.
Results of mixed effects model comparing the anesthetic groups at each time point adjusting for baseline (Model 1)
| Estimated ratio (sevoflurane/xenon) | 95% confidence interval | P-value | |
|---|---|---|---|
| T-tau | |||
| 1 h post-induction | 1.15 | 0.71–1.85 | 0.574 |
| 5 h post-induction | 1.08 | 0.66–1.77 | 0.758 |
| 24 h post-induction | 1.40 | 0.85–2.29 | 0.180 |
| P-tau181 | |||
| 1 h post-induction | 1.20 | 0.44–3.23 | 0.714 |
| 5 h post-induction | 0.95 | 0.35–2.60 | 0.921 |
| 24 h post-induction | 0.96 | 0.35–2.61 | 0.927 |
| NFL | |||
| 1 h post-induction | 0.94 | 0.59–1.50 | 0.795 |
| 5 h post-induction | 1.04 | 0.65–1.65 | 0.877 |
| 24 h post-induction | 1.01 | 0.64–1.61 | 0.960 |
Note: The exponentiated coefficient gives the estimated ratio of the concentration of biomarker in the sevoflurane group versus the xenon group at each time point. NFL: Neurofilament light; P-tau181: tau phosphorylated at threonine 181; T-tau: total tau.
Comparison of postoperative biomarker concentration to baseline (Model 2)
The baseline concentration of T-tau was 3.0 pg/mL (median, interquartile range 2.0–3.4 pg/mL). The concentration at 1 hour post-induction was estimated to be 2.19; 95% confidence interval (CI) 1.62–2.95; P < 0.001, times greater than at baseline (Figure 2 and Table 3). The concentration at 5 and 24 hours post-induction was statistically similar to baseline values.
Table 3.
Results of mixed effects model comparing the concentration of biomarker at each timepoint for all participants to baseline values (Model 2)
| Estimated ratio (postoperative time point/baseline) | 95% confidence interval | P-value | |
|---|---|---|---|
| T-tau | |||
| 1 h post-induction | 2.19 | 1.62–2.95 | < 0.001 |
| 5 h post-induction | 1.06 | 0.78–1.43 | 0.716 |
| 24 h post-induction | 1.03 | 0.76–1.39 | 0.854 |
| P-tau181 | |||
| 1 h post-induction | 3.01 | 1.87–4.85 | <0.001 |
| 5 h post-induction | 0.95 | 0.58–1.54 | 0.827 |
| 24 h post-induction | 0.59 | 0.36–0.96 | 0.036 |
| NFL | |||
| 1 h post-induction | 1.10 | 1.00–1.21 | 0.048 |
| 5 h post-induction | 0.92 | 0.84–1.01 | 0.087 |
| 24 h post-induction | 0.94 | 0.86–1.04 | 0.212 |
Note: The exponentiated coefficient gives the estimated ratio of the concentration of biomarker at each timepoint compared to baseline. NFL: Neurofilament light; P-tau181: tau phosphorylated at threonine 181; T-tau: total tau.
The baseline concentration of P-tau181 was 5.8 pg/mL (median, interquartile range 1.9–17.0 pg/mL). The concentration at 1 hour post-induction was estimated to be 3.01; 95% CI 1.87–4.85; P < 0.001, times greater than at baseline. The concentration at 24 hours post-induction was estimated to be significantly lower than at baseline with an estimated ratio of 0.59; 95% CI 0.36– 0.96; P = 0.036. There was no statistically significant difference between baseline and 5-hour values.
The baseline concentration of NFL was 14.0 pg/mL (median, interquartile range 11.2–21.3 pg/mL). The concentration at 1 hour post-induction was estimated to be be 1.10; 95% CI 1.00–1.21; P = 0.048, times greater than at baseline. The concentration at 5 and 24 hours post-induction was not significantly different from baseline.
Cognitive assessment and Brice questionnaire
There was no statistically significant difference in participant MoCA scores between groups at baseline, 5 or 24 hours post-induction (Table 4).
Table 4.
Montreal Cognitive Assessment scores for each anesthetic group
| Xenon (n=11) | Sevoflurane (n=12) | P-value | |
|---|---|---|---|
| Preoperative | 26.5±2.4 | 24.3±3.9 | 0.14 |
| 5 h post-induction | 25.4±2.0 | 23.6±2.9 | 0.11 |
| 24 h post-induction | 26.4±2.0 | 25.8±2.7 | 0.53 |
Note: Data are presented as mean ± SD, and were analyzed by Student’s t-test.
Two participants in the sevoflurane group could not be contacted to administer the Brice questionnaire. All other participants completed the questionnaire. No participants in the xenon or sevoflurane group reported either memories or dreams between induction and emergence from anesthesia.
DISCUSSION
In the present study, there was no statistically significant difference in the concentrations of the neural injury biomarkers T-tau and NFL in participants who received xenon or sevoflurane anesthesia within 24 hours post-induction. There was also no difference between groups in the concentration of P-tau181 within 24 hours post-induction. We did find that there was a statistically significant increase in the concentration of all three biomarkers following anesthesia at 1 hour post-induction when compared to baseline.
Whilst we are unaware of any previous studies that have compared the impact of different general anesthetic agents on the release of the neural injury biomarkers T-tau and NFL, previous studies have investigated changes in these biomarkers following anesthesia and surgery.14,15,16,17 To the authors’ knowledge, no previous study has investigated the perioperative changes in P-tau181, a marker of tau phosphorylation.
An increase in plasma levels of both T-tau and NFL was observed in a study of 30 participants aged 60 years and older who received either inhalational or intravenous general anesthesia. The majority of participants underwent orthopedic surgery, while six patients underwent cardiac surgery.14 An increase in T-tau was identified 1 hour following surgical incision. However, in contrast to the present study, the plasma level of tau continued to rise beyond 1 hour and peaked at the next sampling time point, 6 hours post-incision.
This earlier study also identified a significant increase in NFL following anesthesia and surgery with a mean peak increase 67% above baseline. The authors found that NFL values were trending upward at the final sampling time point, 48 hours post-incision. In keeping with this, Casey and colleagues study of non-cardiac surgery showed that NFL continues to rise up to 4 days postoperatively.15 Whilst a small but statistically significant increase in NFL was detected at 1 hour post-induction in our own study, the concentration at 5 and 24 hours post-induction was similar to baseline. It may be that our failure to identify an increase in T-tau or NFL at the 5- and 24-hour time points was due to a comparatively reduced surgical and anesthetic insult.
Another recent study utilized a participant cohort who received anesthesia in the absence of surgery in an attempt to distinguish between the effects of anesthesia and surgery on neural biomarker release.17 In this study 59 participants received a 2-hour inhalational anesthetic for an imaging procedure with no surgical stimulus. The authors reported that there was a decrease in three neural biomarkers, T-tau, NFL and glial fibrillary acid protein, 5 hours following anesthesia induction. This was the only post-induction time point measured. The authors concluded that increases in neural biomarkers found in other studies are likely related to the surgical insult rather than exposure to inhalational anesthetic agents.
Our own findings suggest that such a conclusion may be an oversimplification. In the present study, all three biomarkers measured at 5 hours post-induction were not significantly different from baseline, the peak having occurred earlier at 1 hour. Due to the single 5-hour post-induction measurement reported by the previous authors, it remains unclear if anesthesia without surgery would have produced an increase in biomarkers if measured earlier. Our study suggests that a relatively brief anesthetic for a minor interventional procedure, without a skin incision, does result in a significant increase in neural biomarkers.
The study of Casey and colleagues showed that the rise in NFL following a major non-cardiac surgery was greater in participants who developed POD in comparison to those who did not.15 A further analysis of the same cohort indicated that elevated postoperative T-tau was also associated with POD.16 In this second analysis, recovery from delirium was associated with return of T-tau to baseline values whilst NFL continued to rise until day 4 postoperatively. The authors concluded that whilst NFL is likely a marker of neuronal injury, tau protein may indicate more subtle changes in the central nervous system. The authors suggested that elevated tau protein could indicate synaptic pruning of tau containing synpases.16 The significantly greater increase in T-tau as compared to NFL in our own study supports the hypothesis that the presence of tau and NFL in blood plasma could reflect different processes.
This study is the first to consider the effect of anesthesia and surgery on plasma levels of P-tau181, a novel biomarker of AD pathology.9 Anesthetic agents, both inhalational and intravenous, have been shown to induce tau phosphorylation39 and neurons have been shown to metabolize and secrete these phosphorylated forms of tau differently to non-phosphorylated forms.40 The significant increase in P-tau181 postoperatively, a biomarker highly correlated with AD pathology, raises the possibility that perioperative neurocognitive disorders and AD may share pathophysiological mechanisms.
Anesthesia and surgery are associated with the translocation of larger molecules across the blood brain barrier than are permitted in normal physiological circumstances.41,42 It may be that the rise in neural biomarkers in the early postoperative period is a result of translocation from the central nervous system to the periphery rather than an absolute increase in their abundance. The elevation in biomarkers, which was evident within one hour of induction, in our study is not inconsistent with a translocation hypothesis.
In this study, we utilized a surgical cohort that received a highly standardized and relatively minor surgical insult. In this way we anticipated that the contribution of the surgery to the inflammatory and biomarker response would be homogenous, allowing us to attribute any difference in response to the anesthetic. The combined stimulus of the anesthetic and surgery was sufficient to generate a neural biomarker response, and the mean peak increase in tau was in keeping with an earlier study.14
The primary finding of this study is that the use of xenon rather than sevoflurane for general anesthesia did not influence the postoperative release of neural injury biomarkers. Xenon is a potent inhibitor of the N-methyl-D-aspartate (NMDA) receptor whilst sevoflurane, which strongly potentiates γ-aminobutyric acid-A receptors, has little activity at the NMDA receptor.43 Overactivation of the NMDA receptor can occur following acute insults such as stroke, cardiac arrest and traumatic brain injury33 as well as in chronic neurodegenerative diseases such as AD.44 Xenon is thought to provide neuroprotection through NMDA antagonism and it lacks the intrinsic neurotoxic properties of other NMDA antagonists such as ketamine.33
An alternative mechanism for the reduction in postoperative complications with xenon anesthesia is an improvement in end-organ perfusion due to greater hemodynamic stability.26,33 Participants in the xenon group in our study maintained a mean arterial pressure significantly closer to their baseline and received significantly less ephedrine than those in the sevoflurane group (Table 1). Despite this, the release in neural biomarkers was similar in both groups. This would suggest that in the setting of a minor procedure, the greater hemodynamic stability achieved with xenon does not contribute to a reduction in neural injury biomarker release.
Xenon has a mechanistic basis for superior neuroprotection and animal studies have identified neuroprotection in response to a wide variety of insults.25 There are also encouraging findings from a pilot study of xenon versus hypothermia alone following out of hospital cardiac arrest.33 Despite this, xenon as an anesthetic agent has not shown an ability to improve postoperative cognitive outcomes.30,32 In keeping with this we found no difference in MoCA scores between anesthetic groups at each postoperative time point.
It may be that the mechanisms of neurological injury related to anesthesia and surgery are different to those related to hypoxic-ischemic injury in which the preclinical evidence for xenon neuroprotection is strongest.25 Our own findings suggest that one proposed mechanism underlying xenon neuroprotection in the perioperative setting, namely reduced neuronal cell death, is not reflected in reduced neural injury biomarkers in the perioperative period.
There are several limitations with this study. Given the use of a relatively novel anesthetic agent, it was deemed inappropriate to recruit high-risk (ASA III and IV) patients and the inclusion criteria allowed for patients as young as 50 years old. The relatively low incidence of postoperative cognitive dysfunction amongst this patient group limited the ability to identify differences in clinical outcomes between the groups. Postoperative assessment of cognitive function was limited to the MoCA, a tool primarily utilized for the detection of mild cognitive impairment. The power calculation was based on biomarker results from a previous study involving a greater duration of anesthesia and a more invasive surgical procedure.14 Despite this, there was a similar mean peak increase in tau protein (257% vs. 235%) in the two studies. However, this increase was not sustained beyond 1 hour in our study and the rise in NFL observed in our cohort was lower than that described in the previous study.
In conclusion, we identified that there was no difference in the measurements of neural injury biomarkers between participants receiving either xenon or sevoflurane anesthesia following a minor, non-invasive interventional procedure. There was, however, a significant increase in T-tau, P-tau181 and NFL at 1 hour post-anesthesia induction which then returned to baseline. General anesthesia in conjunction with a minor procedure was associated with significant early increases in plasma levels of neural injury biomarkers.
Acknowledgements
We thank Dr. Roman Kluger, St. Vincent’s Hospital, Melbourne and Cameron Patrick, University of Melbourne for their assistance with statistical analysis; Babak Shoghi, Biomedical Engineering, St. Vincent’s Hospital Melbourne for his support and expertise in managing the xenon delivery apparatus; Petrea Corcoran, Anesthetic Research Nurse, St. Vincent’s Hospital Melbourne for her assistance in data collection and as a valuable liaison with participants; Erika Fortunato, Clinical Research Assistant, St. Vincent’s Hospital Melbourne for her assistance in data management, data collection and general enthusiasm for the process; Dr. Alex Bolger, MBBS, St. Vincent’s Hospital Melbourne, Anesthesia resident for her assistance in data collection and participant liaison; Sharon Keys, Lithotripsy coordinator, St. Vincent’s Hospital Melbourne for her invaluable assistance in participant recruitment; Mark Salvatore, Lithotripsy Radiographer and Mark Pemberton-Webb, Lithotripsy Technician, St. Vincent’s Hospital Melbourne for their patience and professionalism.
Footnotes
Conflicts of interest
HZ has served at scientific advisory boards for Alector, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. The other authors (SM, LE, DS, BS) declare no competing interests.
Financial support
This study was supported in part by grants from the ANZCA Research Foundation, Australian and New Zealand College of Anesthetists [N20/009, AMEARA/19].
Institutional review board statement
The protocol was approved by the St. Vincent’s Hospital Melbourne Ethics Committee (approval No. HREC/18/SVHM/221) on July 20, 2018 and was registered with the Australia New Zealand Clinical Trials Registry (registration No. ACTRN12618000916246) on May 31, 2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have consented to their clinical information being reported in an academic journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement
This study follows the CONsolidated Standards Of Reporting Trials (CONSORT) statement for protocol reporting.
Biostatistics statement
The statistical methods of this study were reviewed by Cameron Patrick, Statistical Consultant of The University of Melbourne, Australia.
Copyright transfer agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Individual participant data that underlie these results can be shared following deidentification. The study protocol will also be made available on request. Data will be available from 9 months until 36 months after publication. Data can be shared with investigations whose proposed use has been approved by an independent review committee and after approval by the Research Governance Unit of St. Vincent’s Hospital, Melbourne. Proposals should be directed to steven.mcguigan@svha.org.au. To gain access data requestors will need to sign a data transfer agreement.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Funding: This study was supported in part by grants from the Australian and New Zealand College of Anaesthetists (ANZCA) Research Foundation, Australian and New Zealand College of Anesthetists [N20/009, AMEARA/19].
REFERENCES
- 1.Belrose JC, Noppens RR. Anesthesiology and cognitive impairment: a narrative review of current clinical literature. BMC Anesthesiol. 2019;19:241. doi: 10.1186/s12871-019-0903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma D, Williamson P, Januszewski A, et al. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746–753. doi: 10.1097/01.anes.0000264762.48920.80. [DOI] [PubMed] [Google Scholar]
- 3.Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol. 2008;18:198–210. doi: 10.1111/j.1750-3639.2007.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong Y, Zhang G, Zhang B, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66:620–631. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhen Y, Dong Y, Wu X, et al. Nitrous oxide plus isoflurane induces apoptosis and increases beta-amyloid protein levels. Anesthesiology. 2009;111:741–752. doi: 10.1097/ALN.0b013e3181b27fd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittington RA, Virág L, Marcouiller F, et al. Propofol directly increases tau phosphorylation. PLoS One. 2011;6:e16648. doi: 10.1371/journal.pone.0016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silbert BS, Evered LA, Scott DA. Incidence of postoperative cognitive dysfunction after general or spinal anaesthesia for extracorporeal shock wave lithotripsy. Br J Anaesth. 2014;113:784–791. doi: 10.1093/bja/aeu163. [DOI] [PubMed] [Google Scholar]
- 8.Miller D, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2018;8:Cd012317. doi: 10.1002/14651858.CD012317.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422–433. doi: 10.1016/S1474-4422(20)30071-5. [DOI] [PubMed] [Google Scholar]
- 10.Thomann PA, Kaiser E, Schönknecht P, Pantel J, Essig M, Schröder J. Association of total tau and phosphorylated tau 181 protein levels in cerebrospinal fluid with cerebral atrophy in mild cognitive impairment and Alzheimer disease. J Psychiatry Neurosci. 2009;34:136–142. [PMC free article] [PubMed] [Google Scholar]
- 11.Liliang PC, Liang CL, Weng HC, et al. Tau proteins in serum predict outcome after severe traumatic brain injury. J Surg Res. 2010;160:302–307. doi: 10.1016/j.jss.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Al Nimer F, Thelin E, Nyström H, et al. Comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PLoS One. 2015;10:e0132177. doi: 10.1371/journal.pone.0132177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattsson N, Zetterberg H, Nielsen N, et al. Serum tau and neurological outcome in cardiac arrest. Ann Neurol. 2017;82:665–675. doi: 10.1002/ana.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evered L, Silbert B, Scott DA, Zetterberg H, Blennow K. Association of changes in plasma neurofilament light and tau levels with anesthesia and surgery: results from the CAPACITY and ARCADIAN Studies. JAMA Neurol. 2018;75:542–547. doi: 10.1001/jamaneurol.2017.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey CP, Lindroth H, Mohanty R, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballweg T, White M, Parker M, et al. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br J Anaesth. 2021;126:458–466. doi: 10.1016/j.bja.2020.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deiner S, Baxter MG, Mincer JS, et al. Human plasma biomarker responses to inhalational general anaesthesia without surgery. Br J Anaesth. 2020;125:282–290. doi: 10.1016/j.bja.2020.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26:379–386. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- 19.Whittington RA, Bretteville A, Dickler MF, Planel E. Anesthesia and tau pathology. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:147–155. doi: 10.1016/j.pnpbp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Run X, Liang Z, Zhang L, Iqbal K, Grundke-Iqbal I, Gong CX. Anesthesia induces phosphorylation of tau. J Alzheimers Dis. 2009;16:619–626. doi: 10.3233/JAD-2009-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Freche H, Brouillette J, Fernandez-Gomez FJ, et al. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology. 2012;116:779–787. doi: 10.1097/ALN.0b013e31824be8c7. [DOI] [PubMed] [Google Scholar]
- 22.Barthélemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med. 2020;217:e20200861. doi: 10.1084/jem.20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006;63:1277–1280. doi: 10.1001/archneur.63.9.1277. [DOI] [PubMed] [Google Scholar]
- 24.Cattano D, Williamson P, Fukui K, et al. Potential of xenon to induce or to protect against neuroapoptosis in the developing mouse brain. Can J Anaesth. 2008;55:429–436. doi: 10.1007/BF03016309. [DOI] [PubMed] [Google Scholar]
- 25.Van Hese L, Al Tmimi L, Devroe S, Sanders RD, Fieuws S, Rex S. Neuroprotective properties of xenon in different types of CNS injury. Br J Anaesth. 2018;121:1365–1368. doi: 10.1016/j.bja.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Nair AS, Christopher A, Pulipaka SK, Suvvari P, Kodisharapu PK, Rayani BK. Efficacy of xenon anesthesia in preventing postoperative cognitive dysfunction after cardiac and major non-cardiac surgeries in elderly patients: a topical review. Med Gas Res. 2021;11:110–113. doi: 10.4103/2045-9912.314330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui RS, Wang K, Wang ZL. Sevoflurane anesthesia alters cognitive function by activating inflammation and cell death in rats. Exp Ther Med. 2018;15:4127–4130. doi: 10.3892/etm.2018.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue H, Xu Y, Wang S, et al. Sevoflurane post-conditioning alleviates neonatal rat hypoxic-ischemic cerebral injury via Ezh2-regulated autophagy. Drug Des Devel Ther. 2019;13:1691–1706. doi: 10.2147/DDDT.S197325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neag MA, Mitre AO, Catinean A, Mitre CI. An overview on the mechanisms of neuroprotection and neurotoxicity of isoflurane and sevoflurane in experimental studies. Brain Res Bull. 2020;165:281–289. doi: 10.1016/j.brainresbull.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Coburn M, Sanders RD, Maze M, et al. The hip fracture surgery in elderly patients (HIPELD) study to evaluate xenon anaesthesia for the prevention of postoperative delirium: a multicentre, randomized clinical trial. Br J Anaesth. 2018;120:127–137. doi: 10.1016/j.bja.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Al Tmimi L, Van Hemelrijck J, Van de Velde M, et al. Xenon anaesthesia for patients undergoing off-pump coronary artery bypass graft surgery: a prospective randomized controlled pilot trial. Br J Anaesth. 2015;115:550–559. doi: 10.1093/bja/aev303. [DOI] [PubMed] [Google Scholar]
- 32.Al Tmimi L, Verbrugghe P, Van de Velde M, et al. Intraoperative xenon for prevention of delirium after on-pump cardiac surgery: a randomised, observer-blind, controlled clinical trial. Br J Anaesth. 2020 doi: 10.1016/j.bja.2019.11.037. doi: 10.1016/j.bja.2019.11.037. [DOI] [PubMed] [Google Scholar]
- 33.Maze M, Laitio T. Neuroprotective properties of xenon. Mol Neurobiol. 2020;57:118–124. doi: 10.1007/s12035-019-01761-z. [DOI] [PubMed] [Google Scholar]
- 34.McGuigan S, Evered L, Silbert B, et al. Comparison of the Spectral Features of the Frontal Electroencephalogram in Patients Receiving Xenon and Sevoflurane General Anesthesia. Anesth Analg. 2021 doi: 10.1213/ANE.0000000000005608. doi: 10.1213/ANE.0000000000005608. [DOI] [PubMed] [Google Scholar]
- 35.Law LS, Lo EA, Gan TJ. Xenon anesthesia: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2016;122:678–697. doi: 10.1213/ANE.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 36.Nakata Y, Goto T, Ishiguro Y, et al. Minimum alveolar concentration (MAC) of xenon with sevoflurane in humans. Anesthesiology. 2001;94:611–614. doi: 10.1097/00000542-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013;9:529–537. doi: 10.1016/j.jalz.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brice DD, Hetherington RR, Utting JE. A simple study of awareness and dreaming during anaesthesia. Br J Anaesth. 1970;42:535–542. doi: 10.1093/bja/42.6.535. [DOI] [PubMed] [Google Scholar]
- 39.Tao G, Zhang J, Zhang L, et al. Sevoflurane induces tau phosphorylation and glycogen synthase kinase 3β activation in young mice. Anesthesiology. 2014;121:510–527. doi: 10.1097/ALN.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato C, Barthélemy NR, Mawuenyega KG, et al. Tau kinetics in neurons and the human central nervous system. Neuron. 2018;97:1284–1298. doi: 10.1016/j.neuron.2018.02.015. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang S, Gu C, Mandeville ET, et al. Anesthesia and surgery impair blood-brain barrier and cognitive function in mice. Front Immunol. 2017;8:902. doi: 10.3389/fimmu.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brosnan RJ, Thiesen R. Increased NMDA receptor inhibition at an increased Sevoflurane MAC. BMC Anesthesiol. 2012;12:9. doi: 10.1186/1471-2253-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewerenz J, Maher P. Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Front Neurosci. 2015;9:469. doi: 10.3389/fnins.2015.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]


