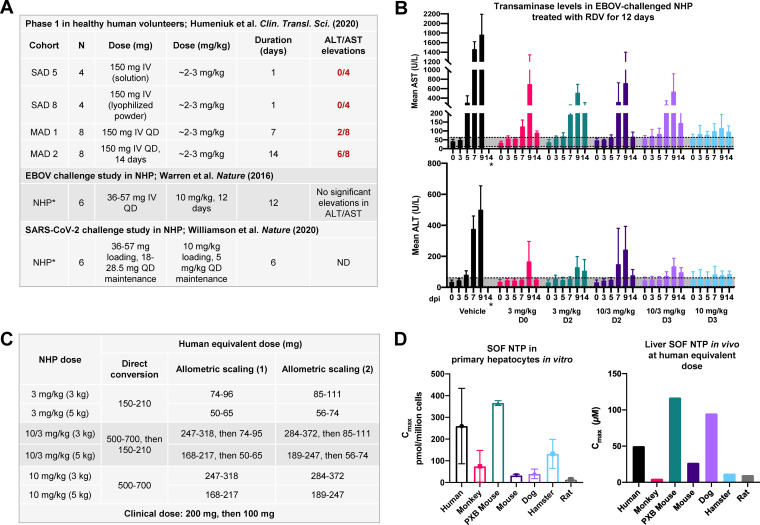
FIG 3.
Humans exhibit higher hepatic extraction of McGuigan prodrugs than nonhuman primates. (A) Doses of RDV trialed in the single ascending dose (SAD) and multiple ascending dose (MAD) arms of the phase 1 trial for a 50- to 70-kg human compared to the dose administered in a 3.6- to 5.7-kg NHP. The human and NHP doses have been converted to milligrams per kilogram and milligrams, respectively. Values were obtained from references 8, 30, and 11. (B) ALT and AST levels in NHP (rhesus macaques; 6 per group) challenged with EBOV and treated with RDV for a total of 12 days (adapted from reference 8). Regions shaded in gray correspond to normal ALT/AST ranges in NHP (ALT,5 to 61 U/liter; AST, 12 to 63 U/liter) (56). NHPs treated at 10 mg/kg for 12 days (light blue) did not experience significant elevations in ALT/AST. *, No ALT/AST values were obtained at 14 days postinfection (dpi) in vehicle control animals because 100% of animals had died. (C) Human equivalent dose (HED) of NHP RDV doses tested in reference 8 via direct conversion and allometric scaling as described in reference 40. Allometric scaling (1) uses an exponent of 0.75, while allometric scaling (2) uses an exponent of 0.80. HEDs were calculated for rhesus macaques weighing 3 kg (light gray) and 5 kg (dark gray) using the Clymer allometric scaling calculator. (D) Sofosbuvir (SOF) is another McGuigan prodrug that is more readily metabolized in PHH than primary monkey hepatocytes in vitro (left) and in vivo (right). (Left) Levels of SOF NTP in primary hepatocytes following a 2-h pulse of 10 μM SOF. (Right) Levels of SOF NTP in human explanted livers and in preclinical model species at the allometrically scaled human dose (400 mg). Open bars, Cmax; shaded bars, C24. C24 in primary monkey livers was below the detection threshold. Values were replotted from references 43 and 42. Formation of SOF NTP is higher in PHH than primary monkey hepatocytes in vitro and in vivo, suggesting more efficient metabolism of McGuigan prodrugs in human than monkey livers.