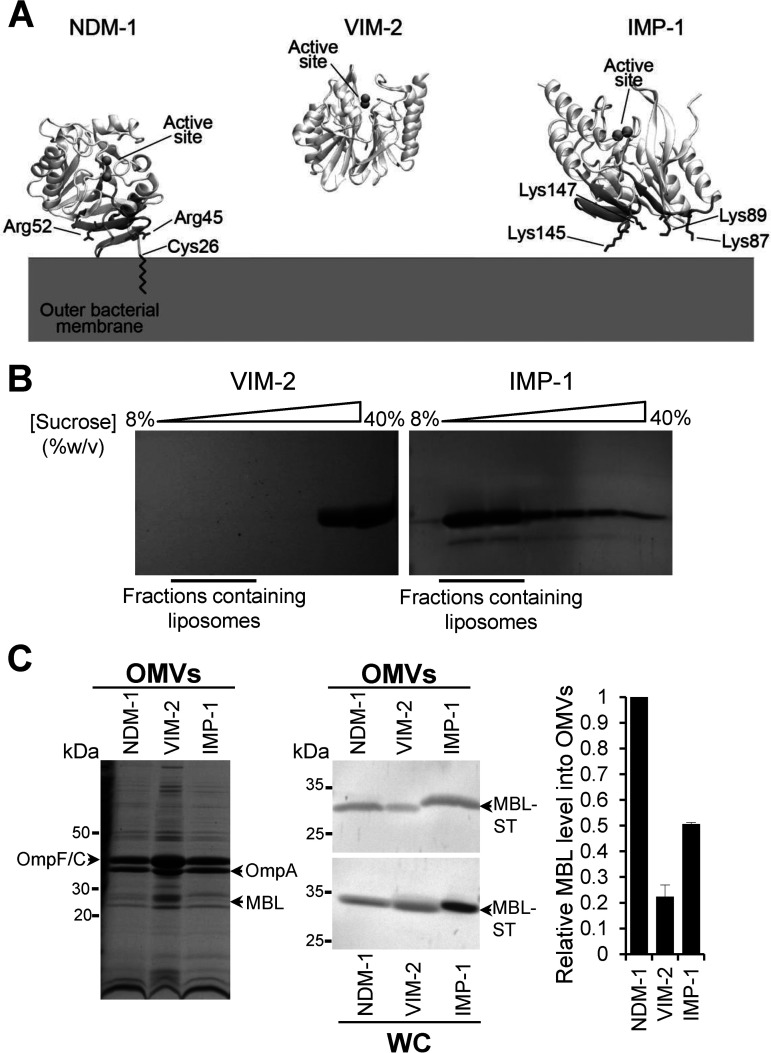
FIG 2.
NDM-1, IMP-1, and VIM-2 MBLs have different affinities for the bacterial membrane that impact on the protein cargo into vesicles. (A) Identified surfaces of interaction between NDM-1, VIM-2, and IMP-1 and the bacterial outer membrane. The positively charged residues are in sticks. VIM-2 did not interact with the membrane in any of the MD replicas and, therefore, does not present an interaction patch. (B) SDS-PAGE analysis of sucrose gradient fractions from liposome flotation assays of VIM-2 and IMP-1. The flotation assays were carried out using liposomes made with an outer membrane composition from E. coli. (C) SDS-PAGE analysis (left) and immunoblot analysis (middle) of OMVs and whole cells (WC) from E. coli expressing NDM-1, VIM-2, or IMP-1, after induction with 20 μM IPTG. (C, Right) Comparison between levels of NDM-1, VIM-2, and IMP-1 into OMVs. The plotted values, relativized to the NDM-1 values, were obtained as described in Materials and Methods. Data correspond to two independent experiments and are shown as the mean value. Error bars represent the standard deviation (SD).