ABSTRACT
The objectives of this study were to evaluate the pattern of antibiotic prescription for endodontic infections (EIs) among Italian dental practitioners (DPs) and to explore the role of potential predictors of antibiotic overprescription. A nationwide cross-sectional survey was conducted between 1 April and 30 October 2019 using a structured questionnaire. Information was gathered on demographics, professional characteristics, and practices regarding antibiotic prescription for both therapeutic and prophylactic purposes. Of the 1,250 invited DPs, 563 answered the general questionnaire (response rate of 52.6%). The proportions of DPs who prescribed an antibiotic without indication for therapeutic and prophylactic purposes were 33.3% and 30.2%, respectively. Acute alveolar abscess without systemic involvement represents the clinical scenario at a high risk of overprescription for therapeutic purposes. Possible predictors of overprescribing included demographics and professional characteristics. Moreover, overprescribing was found to be higher for EIs without an indication than for the cases in which the prescription is indicated for therapeutic purposes. The odds of overprescription for prophylactic purposes were higher for cases of acute apical periodontitis and lower for cases of symptomatic irreversible pulpitis than for acute and chronic alveolar abscesses, for which prescription is indicated. The main findings of the present study provide up-to-date insight into the pattern of antibiotic prescriptions for EIs and evidence useful to identify opportunities to reduce overprescription among DPs through tailored interventions. The development of practical antibiotic prescribing guidelines with a clear description of indications and regimen ease of use is strongly needed.
KEYWORDS: antibiotic prescription, antimicrobial resistance, appropriateness, dental practitioners, dentistry, Italy
INTRODUCTION
Antibiotics are one of the most cost-effective life-saving medical interventions, and their use has not only resulted in saving lives but also contributed to an extended life span (1). Today, the irrational use of antibiotics, combined with the lack of novel antibiotics in the pipeline, is one of the major contributors to the rapid growth of antibiotic resistance (ABR), a widely acknowledged threat to global health (2). According to the World Health Organization (WHO) definition, drugs are used “rationally” when patients receive the appropriate medicines, for appropriate indications, in doses that meet their individual requirements, for an adequate period of time, at the lowest cost to both them and society, and with appropriate information. “Irrational” or unnecessary use of medicines occurs when one or more of these conditions are not met (3).
To adequately address the threat of ABR, it is essential to understand the main factors driving inappropriate antibiotic use in order to design and implement effective actions to improve the use of antibiotics and, ultimately, minimize ABR (4). Prescribers are responsible for making the decision to use antibiotics and for the selection of the type of antibiotic (5). However, in a recent systematic review, it was reported that physicians generally believed that ABR was a serious problem but not in their proximity (6). Among clinicians, it has been estimated that each dental practitioner (DP) could be prescribing 159 antibiotic courses each year, an average of three prescriptions a week, implying considerable antibiotic use (7), which could have a potentially significant impact on ABR (8). A number of studies have shown that the empirical prescription of antibiotics is widespread in dental practices (9–11). More information regarding the appropriateness of antibiotic prescribing in this area is strongly needed. Therefore, the primary objectives of this study were to evaluate the pattern of antibiotic prescription for endodontic infections (EIs) among Italian DPs and adherence to evidence-based recommendations and to explore the role of potential predictors of antibiotic overprescription. The secondary objectives were to assess antibiotic prescription for prophylaxis of serious distant-site infections in EI and whether it complied with evidence-based recommendations.
RESULTS
Sociodemographic characteristics and clinical information-seeking behaviors of DPs.
Of the 1,250 selected DPs invited to participate in the study, 81 were ineligible because they did not practice dental care or had moved abroad, and 98 were not included because of incorrect e-mail addresses. A total of 563 DPs answered the general questionnaire, giving a response rate of 52.6%. The sociodemographic characteristics and clinical information-seeking behaviors of DPs are displayed in Table 1. The majority of respondents were males (71.2%), and the median age was 53 years (interquartile range [IQR], 41 to 60 years). Among the enrolled DPs, 34.8% were from the northwest area, 30.7% were from the south, 13.7% were from the center, 13.3% were from the northeast, and 7.5% were from the islands. The majority of participants (60.9%) had a dentistry degree. The median number of years in practice was 25 (IQR, 13 to 31 years), with a minimum of 1 and a maximum of 44 years. More than half of the DPs self-reported that they advise patients about the antibiotic regimen (60.9%) and about the consequences of nonadherence to therapy (83.5%). About two-thirds (67.5%) asked about any history of high-risk conditions, and 81.9% inquired about antibiotic use in the previous week. More than half (55.4%) of the participants reported to be knowledgeable about guidelines on the use of systemic antibiotics in endodontics, and 79.2% declared that they needed further information on the topic.
TABLE 1.
Sociodemographic characteristics and clinical information-seeking behaviors of dental practitioners
| DP characteristic (no. of DPs responding to question) | Value |
||
|---|---|---|---|
| No. | % | Median (IQR) | |
| Gender (556) | |||
| Male | 396 | 71.2 | |
| Female | 160 | 28.8 | |
| Age (yrs) (529) | 53 (41–60) | ||
| Residence (549) | |||
| Northwest | 191 | 34.8 | |
| South | 169 | 30.7 | |
| Center | 75 | 13.7 | |
| Northeast | 73 | 13.3 | |
| Islands | 41 | 7.5 | |
| Academic degree (557) | |||
| Medicine | 218 | 39.1 | |
| Dentistry | 339 | 60.9 | |
| No. of yrs in practice (545) | 25 (13–31) | ||
| Advising patients about antibiotic regimen (563) | |||
| No | 220 | 39.1 | |
| Yes | 343 | 60.9 | |
| Advising patients about consequences of nonadherence to therapy (563) | |||
| No | 93 | 17.4 | |
| Yes | 470 | 83.5 | |
| Asking about history of high-risk conditions (563) | |||
| No | 137 | 24.3 | |
| Yes | 426 | 75.7 | |
| Asking about antibiotic use in the previous wk (563) | |||
| No | 102 | 18.1 | |
| Yes | 461 | 81.9 | |
| Knowledge of guidelines (563) | |||
| No | 251 | 44.6 | |
| Yes | 312 | 55.4 | |
| Need for more information on the topic (563) | |||
| No | 117 | 20.8 | |
| Yes | 446 | 79.2 | |
Descriptive results regarding antibiotic prescription for therapeutic purposes.
The overall proportion of DPs who prescribed an antibiotic without indication for therapeutic purposes was 33.3%. Antibiotic prescriptions according to EIs and the presence of an indication are shown in Table 2. The proportions of DPs who prescribed an antibiotic without indication ranged from 13% for irreversible symptomatic pulpitis to 62% for acute alveolar abscess without systemic involvement. In accordance with evidence-based recommendations, the antibiotic was prescribed in 78.3% of cases of acute alveolar abscess with systemic involvement. The DPs usually recommended a treatment duration of 5 to 6 days (47.2%) or 2 to 3 days (28.3%), with a median duration of antibiotic therapy of 6 days (IQR, 3 to 6 days). For patients who did not report a penicillin allergy, the combination of amoxicillin plus clavulanate was the most frequently prescribed antibiotic in all explored EIs, ranging from 46.4% for symptomatic irreversible pulpitis to 58.9% for acute apical periodontitis. Amoxicillin was less frequently prescribed than amoxicillin plus clavulanate, ranging from 28.5% for acute alveolar abscesses with systemic involvement to 43.8% for symptomatic irreversible pulpitis. A high proportion of DPs (74.1%) self-reported clindamycin prescription in penicillin-allergic patients, according to suggestions of evidence-based recommendations.
TABLE 2.
Pattern of antibiotic prescription in endodontic infections according to purpose and the presence of an indication
| Purpose | No. of responses or median value (IQR) | % of responses |
|---|---|---|
| Therapeutic | ||
| Without indication | ||
| Chronic alveolar abscess | 121 | 21.5 |
| Acute alveolar abscess without systemic involvement | 349 | 62 |
| Symptomatic irreversible pulpitis | 73 | 13 |
| Acute apical periodontitis | 242 | 43 |
| Pulp necrosis | 152 | 27 |
| With indication | ||
| Acute alveolar abscess with systemic involvement | 441 | 78.3 |
| Duration (days) | 6 (3–6) | |
| Prophylactica | ||
| Without indication | ||
| Symptomatic irreversible pulpitis | 297 | 17.6 |
| Acute apical periodontitis | 765 | 45.3 |
| Pulp necrosis | 466 | 27.6 |
| With indication | ||
| Chronic alveolar abscess | 1,137 | 67.3 |
| Acute alveolar abscess without systemic involvement | 1,019 | 60.3 |
| Acute alveolar abscess with systemic involvement | 1,131 | 67 |
| Timingb | ||
| Appropriate (30–60 min before the procedure) | 400 | 76.6 |
| Inappropriate (24–48 h before the procedure) | 122 | 23.4 |
| Durationb | ||
| Appropriate (within 24 h from the procedure) | 487 | 93.3 |
| Inappropriate (over 24 h from the procedure) | 35 | 6.7 |
The total number of responses to the questions exceeds 563 since each DP could answer a question more than once with regard to three high-risk clinical conditions (i.e., those patients with a previous diagnosis of infective endocarditis, those with a prosthetic valve or prosthetic material used for cardiac valve repair, and those patients with a replacement of a joint prosthesis in the previous 6 months), and the percentages were calculated by dividing the absolute frequency by the total number of responses (n = 1,689).
The number of DPs responding to the question is 522.
Mixed-effects logistic regression model results regarding antibiotic prescription for therapeutic purposes.
Regarding possible predictors of overprescribing for therapeutic purposes, it was less likely among the DPs with a dentistry degree (odds ratio [OR] = 0.59 [95% confidence interval {CI} = 0.41 to 0.86]) than among those with a medical degree and for those who had attended continuing education courses (OR = 0.75 [95% CI = 0.58 to 0.96]) than for those who had used other sources of information. Furthermore, the 41- to 60-year age groups were positively associated with antibiotic overprescription compared with the >60-year-old DPs, and the effect size for the 41- to 50-year age group (OR = 2.26 [95% CI = 1.36 to 3.75]) was higher than that for the 51- to 60-year age group (OR = 1.65 [95% CI = 1.12 to 2.42]). Having advised the patient about an antibiotic regimen was not associated with correct prescription practice (OR = 0.63 [95% CI = 0.49 to 0.81]). As expected, for all but one of the EIs without indication (i.e., symptomatic irreversible pulpitis), the rate of antibiotic prescription was found to be higher than in the cases of acute alveolar abscess with systemic involvement, for which the prescription was indicated. In particular, higher odds of overprescription for therapeutic purposes were found in cases of acute alveolar abscess without systemic involvement (OR = 13.32 [95% CI = 9.22 to 19.23]), acute apical periodontitis (OR = 5.11 [95% CI = 3.6 to 7.24]), and pulp necrosis (OR = 2.23 [95% CI = 1.56 to 3.18]) (model 1 in Table 3). The receiver operating characteristic (ROC) analysis reveals good discrimination (area under the curve [AUC] = 0.82 [95% CI = 0.80 to 0.84]), and no outliers were detected by Pearson’s residual analysis.
TABLE 3.
Mixed-effects logistic regression model results for potential determinants of the different outcomes of interest
| Variablea | OR | 95% CI | P |
|---|---|---|---|
| Model 1 (outcome: antibiotic overprescription for therapeutic purposes) (log likelihood = −12,762.714; P > χ2 = 0.0000; observations = 2,394) | |||
| Gender | |||
| Maleb | 1.00 | ||
| Female | 0.99 | 0.76–1.29 | 0.946 |
| Age, ordinal (yrs) | |||
| ≤40 | 1.38 | 0.84–2.26 | 0.206 |
| 41–50 | 2.26 | 1.36–3.75 | 0.002 |
| 51–60 | 1.65 | 1.12–2.42 | 0.011 |
| >60b | 1.00 | ||
| College degree | |||
| Medicineb | 1.00 | ||
| Dentistry | 0.59 | 0.41–0.86 | 0.005 |
| Sources of information | |||
| Colleagues/Internet/scientific journalsb | 1.00 | ||
| Continuing education courses | 0.75 | 0.58–0.96 | 0.020 |
| Knowledge of guidelines | |||
| Nob | 1.00 | ||
| Yes | 1.25 | 0.96–1.62 | 0.102 |
| Advising patients about antibiotic regimen | |||
| Nob | 1.00 | ||
| Yes | 0.63 | 0.49–0.81 | <0.001 |
| Need for more information on the topic | |||
| Nob | 1.00 | ||
| Yes | 0.68 | 0.48–0.96 | 0.027 |
| Endodontic infections | |||
| Acute alveolar abscess with systemic involvementb | 1.00 | ||
| Chronic alveolar abscess | 1.46 | 1.01–2.11 | 0.042 |
| Acute alveolar abscess without systemic involvement | 13.32 | 9.22–19.23 | <0.001 |
| Symptomatic irreversible pulpitis | 0.71 | 0.48–1.06 | 0.102 |
| Acute apical periodontitis | 5.11 | 3.60–7.24 | <0.001 |
| Pulp necrosis | 2.23 | 1.56–3.18 | <0.001 |
| Model 2 (outcome: antibiotic overprescription for prophylactic purposes) (log likelihood = −3,605.3914; P > χ2 = 0.0000; observations = 5,985) | |||
| Gender | |||
| Maleb | 1.00 | ||
| Female | 1.01 | 0.84–1.2 | 0.985 |
| Age, ordinal (yrs) | |||
| ≤40 | 0.90 | 0.64–1.25 | 0.525 |
| 41–50 | 1.07 | 0.76–1.51 | 0.707 |
| 51–60 | 0.95 | 0.73–1.23 | 0.676 |
| >60b | 1.00 | ||
| College degree | |||
| Medicineb | 1.00 | ||
| Dentistry | 0.80 | 0.62–1.04 | 0.095 |
| Sources of information | |||
| Colleagues/Internet/scientific journalsb | 1.00 | ||
| Continuing education courses | 1.04 | 0.88–1.23 | 0.669 |
| Knowledge of guidelines | |||
| Nob | 1.00 | ||
| Yes | 0.97 | 0.81–1.16 | 0.707 |
| Advising patients about antibiotic regimen | |||
| Nob | 1.00 | ||
| Yes | 1.13 | 0.95–1.34 | 0.180 |
| Need for more information on the topic | |||
| Nob | 1.00 | ||
| Yes | 0.92 | 0.73–1.17 | 0.497 |
| Endodontic infections | |||
| Acute alveolar abscess with and without systemic involvement and chronic alveolar abscessb | 1.00 | ||
| Symptomatic irreversible pulpitis | 0.49 | 0.41–0.58 | <0.001 |
| Acute apical periodontitis | 2.13 | 1.83–2.47 | <0.001 |
| Pulp necrosis | 0.91 | 0.78–1.06 | 0.208 |
For model 1, the AUC is 0.82 (95% CI = 0.80 to 0.84). For model 2, the AUC is 0.73 (95% CI = 0.72 to 0.74). No outliers were revealed by Pearson residual analysis.
Reference category.
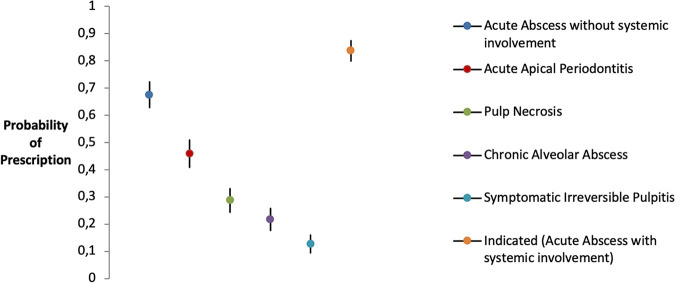
The average predicted probability of prescription at a population level considering a single EI confirms the results of descriptive statistics. Indeed, acute alveolar abscess without systemic involvement represents the clinical scenario at a high risk of overprescription for therapeutic purposes, with a probability (67.5% [95% CI = 62.9 to 72.2%]) significantly higher (P < 0.001) than the average aggregate probability of prescription for all other EIs without an indication (Fig. 1).
FIG 1.
Probabilities of antibiotic prescription for therapeutic purposes according to EIs when not indicated by guidelines. Shown are marginal estimates of prescriptive behavior probabilities predicted at mean values for gender, number of years in practice, geographic area of residence, guideline knowledge, college degree, and advising patients about the antibiotic regimen.
Descriptive results regarding antibiotic prescription for prophylactic purposes.
The overall proportion of DPs who prescribed an antibiotic without an indication for prophylactic purposes was 30.2%. Table 2 shows antibiotic prescriptions according to EIs and the presence of an indication. Among high-risk patients, the proportion of DPs who overprescribed antibiotics (i.e., symptomatic irreversible pulpitis, acute apical periodontitis, and pulp necrosis) was 30.2%, ranging from 17.6% for symptomatic irreversible pulpitis to 45.3% for acute apical periodontitis. When an indication was present, the proportions of DPs who reported prescription were 60.3% for acute alveolar abscesses and 67.3% for chronic alveolar abscesses. The course of antibiotic prophylaxis prescribed by DPs was consistent with guidelines (i.e., a single dose of amoxicillin 1 h before the procedure) in 74% of cases.
Mixed-effects logistic regression model results regarding antibiotic prescription for prophylactic purposes.
As shown in model 2 in Table 3, no statistically significant differences were found between antibiotic overprescription for prophylactic purposes and the DPs’ demographic and professional characteristics. With regard to EIs, the odds of overprescription were higher in cases of acute apical periodontitis (OR = 2.13 [95% CI = 1.83 to 2.47]) and lower in cases of symptomatic irreversible pulpitis (OR = 0.49 [95% CI = 0.41 to 0.58]) than in EIs in which the prescription was indicated (i.e., acute alveolar abscess with and without systemic involvement and chronic alveolar abscess). The ROC analysis reveals satisfactory discrimination (AUC = 0.73 [95% CI = 0.72 to 0.74]), and Pearson residual analysis did not show any outliers.
Among high-risk patients, the predicted probability of inappropriate prescription for prophylactic purposes was significantly higher (P < 0.001) in patients with acute apical periodontitis (49.9%) than the average prescription probability of the other two procedures without indication (symptomatic irreversible pulpitis and pulp necrosis) (Fig. 2).
FIG 2.
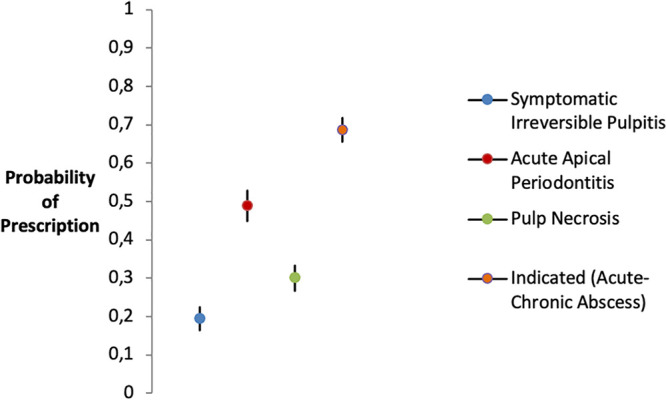
Probabilities of antibiotic prescription for prophylactic purposes according to EIs when not indicated by guidelines. Shown are marginal estimates of prescriptive behavior probabilities predicted at mean values for gender, number of years in practice, geographic area of residence, guideline knowledge, college degree, and advising patients about the antibiotic regimen.
DISCUSSION
To the best of our knowledge, this study represents the first national evaluation of antibiotic prescribing for EIs among DPs. Our goals were to provide up-to-date insight into the pattern of antibiotic prescriptions and to identify opportunities to reduce overprescription through tailored interventions.
This survey provided 3 major findings. First, the results demonstrated that the Italian DPs overprescribed antibiotics in the management of EIs, for both therapeutic and prophylactic purposes. Similar results were found in the outpatient primary medical care setting in the same geographic specialty area (12) as well as in general dental practices, where only 19% of antibiotics were prescribed in situations where their use was indicated by clinical guidelines (13). Interestingly, acute alveolar abscess without systemic involvement is the EI at the highest risk of antibiotic overprescription, with a proportion (62%) slightly lower than that for acute abscess with systemic involvement (78.3%), conditions that definitely require an antibiotic prescription. Studies have shown that adjunctive antibiotics are not effective in preventing or ameliorating signs and symptoms in cases of symptomatic irreversible pulpitis, acute apical periodontitis, or localized acute alveolar abscesses (14–17). Antibiotics should be used only as adjuvant therapies in cases with evidence of systemic involvement following adequate endodontic disinfection and abscess drainage if swelling is present (16–18). As demonstrated in previous studies, DPs routinely prescribe antibiotics for patients with dental pain for the patients’ comfort and to alleviate their apprehension (18). One strategy that may be useful to encourage prudent antibiotic prescription could consider patient education about the signs and symptoms of spreading infection and give the patient a “standby” antibiotic prescription. The patient would fill the prescription and call the prescriber’s office only if he/she perceives the infection to be occurring, prior to receiving definitive care (19). Regarding prophylactic purposes, our results demonstrated that 30.2% of the surveyed DPs reported prescribing antibiotics in high-risk patients but for procedures without manipulation of gingival tissue or the periapical region of the teeth for which there is no evidence of therapeutic benefit (19–22). Indeed, some authors have highlighted that the risk of bacteremia after dental treatment is considered lower than that related to normal daily activities (23–25).
The second key result is that the choices of the antibiotic agent and duration of therapy are typically made in an empirical fashion. It is recommended that when using adjunctive antibiotics in addition to adequate debridement and surgical drainage, such as in cases of spreading infections, the DP should minimize the use of broad-spectrum antibiotics, use the shortest effective course of antibiotics, and monitor the patient closely for the duration of the prescription (19). In the present study, the combination of amoxicillin plus clavulanate was the most frequently prescribed antibiotic, although amoxicillin and penicillin VK should be the first-line therapeutic antibiotics in patients without a penicillin allergy (26). Amoxicillin-clavulanate is recommended only for patients who continue to have an unresolved or recalcitrant infection after treatment with a β-lactam (27). However, a review by Segura-Egea et al. shows in great detail how amoxicillin alone or in combination with clavulanate is the preferred solution for most DPs in Europe (28). Moreover, most DPs prescribe antibiotics in courses of 5 to 6 days (47.2%), similar to findings from previous studies (9, 25). Some evidence suggests that shorter courses (2 to 3 days) may be successfully used as adjuvant therapies (9, 25), and a recent recommendation suggests monitoring a patient’s symptoms daily and discontinuing antibiotics when the symptoms are resolved (9, 11, 19).
Third, antibiotic prescription practice for therapeutic purposes can be shaped by individual characteristics of DPs, such as age, academic degree, and having attended a continuing education course on antibiotic prescriptions. In the present study, younger DPs were more likely to overprescribe than their older colleagues, in contrast to data from other studies that show that young age and a more recent year of graduation were factors independently associated with being worried about liability in antibiotic prescription (29, 30) and the proper use of antibiotics during periodontal therapy. Further work should be done to better clarify the role of the DPs’ age as a potential determinant of overprescription. Moreover, a degree in medicine was found to be associated with the overprescription of antibiotics compared with a degree in dentistry. It should be noted that in Italy, until 1985, dentist status was obtained after completion of training in medicine, with or without the specialty of stomatology. Actually, both stomatologists (M.D.) and odontologists (D.D.S.) practice as dentists in the country. Previous studies showed that physicians appear to prescribe antibiotics for tooth-related conditions more readily than dentists, suggesting that individuals seeing their physician with a dental problem are considerably more likely to receive an antibiotic than those seeing a dentist for similar conditions (31, 32). As expected, the DPs having attended a continuing education course on antibiotic prescriptions were less likely to overprescribe than those who have used other sources of information. Educational interventions to improve antibiotic use are essential, especially if such interventions are active and multifaceted and have been designed to take into account the health professionals’ knowledge and attitudes, in order to focus on the identified barriers (33). Antibiotic prescribing practices are complex processes and associated with both intrinsic (prescriber) and external (patients and institutional environment) factors, and a systematic approach is required to curb the overprescription of antibiotics. It is challenging to change the prescribers’ as well as the public’s stereotype of antibiotics as a panacea. It is equally important, apart from educating prescribers, to educate patients to reduce requests for antibiotics (34) and community pharmacists to avoid dispensing antibiotics without a prescription (35).
Strengths and limitations.
The national level of the study participants and the large sample size represent key strengths of this survey. Having covered the whole country protects from any bias attributable to the prescribing practices of a smaller territory.
Interpretations of our findings should take into account potential limitations. First, the cross-sectional design of the study does not allow conclusions on causality to be drawn about the observed associations. However, this was not our goal since we wanted only to assess the pattern of antibiotic prescriptions in selected EIs and to suggest potential predictors that could influence overprescription by Italian DPs. Second, the method of collecting information can itself be a cause of bias for at least two reasons. First, older dentists may have been less familiar with online questionnaires and could have contributed less to the response rate, and if this is true, we could have overestimated the rates of overprescription since in the present study, older DPs were less likely to overprescribe antibiotics. Second, this is a survey that relied on the DPs’ reporting information. Intentional deception, poor recall, and misunderstanding of the questions can all contribute to a wrong assumption of actual prescribing practice. DPs might report socially acceptable responses that are different from the actual day-to-day practices. However, assurance of confidentiality has substantially minimized this issue in our data. Third, the response rate (52.6%) is lower than the desired response rate, which must be higher than 60%, but we believe that it is satisfactory considering that DPs are a group with very low survey response rates (36–38).
Conclusions.
The main findings of the present study may have implications for future research to identify the DP profile and the EIs most at risk of the prescription of antibiotics without a therapeutic or prophylactic benefit. The development of practical and meaningful antibiotic prescribing guidelines in endodontics with a clear description of indications and regimens to facilitate case selection and ease of use is strongly needed. Evidence from the present study provides some insight into antibiotic prescribing practices and could ultimately contribute to controlling the spread of ABR and limiting unnecessary costs.
MATERIALS AND METHODS
Study design.
This was a nationwide cross-sectional survey based on a structured questionnaire.
Survey sampling methods.
The survey was conducted using a multistage sampling procedure. According to the geographic division of the National Statistical Institute (ISTAT) (39), Italy was divided into five areas (northwest, northeast, central, south, and islands). For each area, a random sample of five Registers of Physicians, Surgeons, and Dentists (RPSDs) was chosen from a publicly available frame of all RPSDs. In Italy, every dentist who practices is obligated to join the RPSD. The chief executive of each selected RSPD was contacted by phone in order to delineate the aims of the study and obtain verbal consent to carry out the study. In cases where permission was refused, we randomly chose another RPSD in the same area, and so forth, until consent was given. Next, starting from 1 April 2019, 50 DPs were randomly chosen among those registered within each selected RPSD to give a total sample of 1,250 DPs. Selected DPs received an e-mail with a link to an electronically administered questionnaire. The link to the questionnaire was personal; it contained a unique serial number but no personal identifiers. Nonrespondents received a reminder after 2 weeks, after 4 weeks, and after 8 weeks, and data collection terminated on 30 October 2019. In an attempt to maximize the response rate, after the three reminders, a phone call was made to the DPs in case they preferred to give their answers via a telephone interview.
Instruments and methods for data collection.
The questionnaire was developed from themes identified in the existing literature and interviews with experienced researchers and DPs. A pilot test was conducted among 10 DPs to assess the relevance and comprehensiveness of the questionnaire battery. Only minor changes (deletion of a few ad hoc items) were made based on the pilot test. Information was gathered on demographics and professional characteristics (gender, age, academic degree, number of years in practice, and place of residence), practices regarding antibiotic prescription for EIs (purpose of prescription, drug choice, and timing and duration of the antibiotic course), and sources of information used to update knowledge on the use of systemic antibiotics in endodontics. Antibiotic prescriptions for therapeutic and prophylactic purposes were explored in cases of acute alveolar abscess with and without systemic involvement, chronic alveolar abscess, symptomatic irreversible pulpitis, acute apical periodontitis, and pulp necrosis. According to guidelines, antibiotic prescription for therapeutic purposes was judged appropriate in only one clinical scenario, i.e., acute apical abscess with systemic involvement (i.e., an elevated body temperature of >38°C, malaise, lymphadenopathy, and trismus) (9, 19). For the purpose of prophylaxis of serious distant-site infections, antibiotic prescription was judged appropriate exclusively in patients classified as being at high risk and in two clinical scenarios, i.e., acute alveolar abscess, both with and without systemic involvement, and chronic alveolar abscess. Indeed, in those EIs, manipulation of the gingival or periapical region of the teeth or perforation of the oral mucosa exposes high-risk patients to a potential serious distant-site infection. In accordance with published guidelines, we considered high-risk patients those with a previous diagnosis of infective endocarditis (IE), patients with a prosthetic valve or prosthetic material used for cardiac valve repair, and patients with a replacement of a joint prosthesis in the previous 6 months (20, 21). Moreover, DPs were asked if the information about patient clinical history and other concomitant treatments was usually investigated as well as if they advised patients about the antibiotic regimen.
Ethics approval was granted by the Calabria Centre Local Human Research Ethics Committee (approval no. 121/2019/04/18).
Statistical analysis.
All collected variables were summarized by means and standard deviations when normally distributed. Medians and interquartile ranges were used in cases of deviations from normality. The skewness of the variables was estimated by Shapiro-Wilk tests. Categorical variables were expressed as percentages. The overall and procedure-specific proportions of antibiotic prescriptions were calculated both with and without indication by the guidelines. The inferential analysis was conducted in two stages. In the first stage, a bivariate analysis was carried out to evaluate the effect of the independent variables on the outcomes of interest using a chi-square test for categorical variables and Student’s t test for continuous variables. The independent variables for which the P value was ≤0.25 upon the bivariate analysis were included in the models (40). In the second stage, since the study had a clustering-data design, a mixed-effects logistic regression model was developed to explore the role of potential predictors of the following outcomes of interest: antibiotic prescription without indication for both therapeutic (model 1) and prophylaxis (model 2) purposes. The following independent variables were included in the models: gender (male = 0; female = 1), age (four categories in years [≤40 = 1; 41 to 50 = 2; 51 to 60 = 3; >60 = 4]), college degree (medicine = 0; dentistry = 1), sources of information (colleagues/Internet/scientific journals = 0; continuing education courses = 1), advising patients about the antibiotic regimen (no = 0; yes = 1), knowledge of guidelines on the use of systemic antibiotics in endodontics (no = 0; yes = 1), and DPs’ perceived need for more information on the topic (no = 0; yes = 1). To take into account the clustered (within-DP) data set structure, the DPs’ identifiers were introduced into the explanatory models as random factors. The goodness of fit of the logistic models was assessed by the AUC test and Pearson residual analysis. Furthermore, in order to highlight in which EIs inappropriate antibiotic prescription particularly emerges, we estimated the average predicted probabilities of antibiotic prescription at a population level for each explored EI. All statistical comparisons were adjusted by Bonferroni correction. Statistical analyses were conducted using the STATA version 16.0 statistical package (StataCorp) (41).
Data availability.
The data set was deposited in the Mendeley Data repository (https://doi.org/10.17632/gbvhzbjnsm.1).
ACKNOWLEDGMENTS
We have no transparency declarations.
This study received no funding.
REFERENCES
- 1.Sengupta S, Chattopadhyay MK, Grossart HP. 2013. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front Microbiol 4:47. 10.3389/fmicb.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ventola CL. 2015. The antibiotic resistance crisis. Part 1: causes and threats. P T 40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Hernandez P, Abegunde D, Edejer T. 2011. The world medicine situation 2011. WHO Press, Geneva, Switzerland. https://www.who.int/medicines/areas/policy/world_medicines_situation/WMS_ch14_wRational.pdf. Accessed 17 March 2021. [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2016. Core elements of outpatient antibiotic stewardship. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/antibiotic-use/community/pdfs/16_268900-A_CoreElementsOutpatient_508.pdf. Accessed 5 June 2021. [Google Scholar]
- 5.European Commission. 2017. EU guidelines for the prudent use of antimicrobials in human health. European Commission, Brussels, Belgium. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.C_.2017.212.01.0001.01.ENG&toc=OJ:C:2017:212:TO. Accessed 28 May 2021. [Google Scholar]
- 6.Machowska A, Stålsby Lundborg C. 2019. Drivers of irrational use of antibiotics in Europe. Int J Environ Res Public Health 16:27. 10.3390/ijerph16010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweeney LC, Dave J, Chambers PA, Heritage J. 2004. Antibiotic resistance in general dental practice—a cause for concern? J Antimicrob Chemother 53:567–576. 10.1093/jac/dkh137. [DOI] [PubMed] [Google Scholar]
- 8.Teoh L, Thompson W, Suda K. 2020. Antimicrobial stewardship in dental practice. J Am Dent Assoc 151:589–595. 10.1016/j.esmoop.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Segura-Egea JJ, Gould K, Şen BH, Jonasson P, Cotti E, Mazzoni A, Sunay H, Tjäderhane L, Dummer PMH. 2018. European Society of Endodontology position statement: the use of antibiotics in endodontics. Int Endod J 51:20–25. 10.1111/iej.12781. [DOI] [PubMed] [Google Scholar]
- 10.Merlos A, Vinuesa T, Jané-Salas E, López-López J, Viñas M. 2014. Antimicrobial prophylaxis in dentistry. J Glob Antimicrob Resist 2:232–238. 10.1016/j.jgar.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Segura-Egea JJ, Gould K, Şen BH, Jonasson P, Cotti E, Mazzoni A, Sunay H, Tjäderhane L, Dummer PMH. 2017. Antibiotics in endodontics: a review. Int Endod J 50:1169–1184. 10.1111/iej.12741. [DOI] [PubMed] [Google Scholar]
- 12.Bianco A, Papadopoli R, Mascaro V, Pileggi C, Pavia M. 2018. Antibiotic prescriptions to adults with acute respiratory tract infections by Italian general practitioners. Infect Drug Resist 11:2199–2205. 10.2147/IDR.S170349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cope AL, Francis NA, Wood F, Chestnutt IG. 2016. Antibiotic prescribing in UK general dental practice: a cross-sectional study. Community Dent Oral Epidemiol 44:145–153. 10.1111/cdoe.12199. [DOI] [PubMed] [Google Scholar]
- 14.Cope AL, Francis N, Wood F, Chestnutt IG. 2018. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Syst Rev 9:CD010136. 10.1002/14651858.CD010136.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry M, Reader AI, Beck M. 2001. Effect of penicillin on postoperative endodontic pain and swelling in symptomatic necrotic teeth. J Endod 27:117–123. 10.1097/00004770-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DC, Sutherland S, Basrani B. 2003. Emergency management of acute apical abscesses in the permanent dentition: a systematic review of the literature. J Can Dent Assoc 69:660. [PubMed] [Google Scholar]
- 17.Pickenpaugh L, Reader A, Beck M, Meyers WJ, Peterson LJ. 2001. Effect of prophylactic amoxicillin on endodontic flare-up in asymptomatic, necrotic teeth. J Endod 27:53–56. 10.1097/00004770-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Aminoshariae A, Kulild JC. 2016. Evidence-based recommendations for antibiotic usage to treat endodontic infections and pain: a systematic review of randomized controlled trials. J Am Dent Assoc 147:186–191. 10.1016/j.adaj.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MD. 2019. Endodontics and antibiotic update. American Association of Endodontists, Chicago, IL. https://f3f142zs0k2w1kg84k5p9i1o-wpengine.netdna-ssl.com/specialty/wp-content/uploads/sites/2/2019/12/ecfe-fall-2019-May-2021.pdf. Accessed 15 February 2021. [Google Scholar]
- 20.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, ESC Scientific Document Group. 2015. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36:3075–3128. 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura RA, Otto CM, Bonow RO, Carabello BA, ErwinJP, III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, SundtTM, III, Thompson A. 2017. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 70:252–289. 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Lockhart PB, Tampi MP, Abt E, Aminoshariae A, Durkin MJ, Fouad AF, Gopal P, Hatten BW, Kennedy E, Lang MS, Patton LL, Paumier T, Suda KJ, Pilcher L, Urquhart O, O’Brien KK, Carrasco-Labra A. 2019. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: a report from the American Dental Association. J Am Dent Assoc 150:906–921.e12. 10.1016/j.adaj.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh Gill A, Morrissey H, Rahman A. 2018. A systematic review and meta-analysis evaluating antibiotic prophylaxis in dental implants and extraction procedures. Medicina (Kaunas) 54:95. 10.3390/medicina54060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suda KJ, Henschel H, Patel U, Fitzpatrick MA, Evans CT. 2018. Use of antibiotic prophylaxis for tooth extractions, dental implants, and periodontal surgical procedures. Open Forum Infect Dis 5:ofx250. 10.1093/ofid/ofx250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dar-Odeh NS, Abu-Hammad OA, Al-Omiri MK, Khraisat AS, Shehabi AA. 2010. Antibiotic prescribing practices by dentists: a review. Ther Clin Risk Manag 6:301–306. 10.2147/tcrm.s9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumgartner JC, Xia T. 2003. Antibiotic susceptibility of bacteria associated with endodontic abscesses. J Endod 29:44–47. 10.1097/00004770-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Salvo F, Polimeni G, Moretti U, Conforti A, Leone R, Leoni O, Motola D, Dusi G, Caputi AP. 2007. Adverse drug reactions related to amoxicillin alone and in association with clavulanic acid: data from spontaneous reporting in Italy. J Antimicrob Chemother 60:121–126. 10.1093/jac/dkm111. [DOI] [PubMed] [Google Scholar]
- 28.Segura-Egea JJ, Martín-González J, Jiménez-Sánchez MDC, Crespo-Gallardo I, Saúco-Márquez JJ, Velasco-Ortega E. 2017. Worldwide pattern of antibiotic prescription in endodontic infections. Int Dent J 67:197–205. 10.1111/idj.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tebano G, Dyar OJ, Beovic B, Béraud G, Thilly N, Pulcini C, ESCMID Study Group for Antimicrobial stewardshiP (ESGAP). 2018. Defensive medicine among antibiotic stewards: the international ESCMID AntibioLegalMap survey. J Antimicrob Chemother 73:1989–1996. 10.1093/jac/dky098. [DOI] [PubMed] [Google Scholar]
- 30.Agossa K, Sy K, Mainville T, Gosset M, Jeanne S, Grosgogeat B, Siepmann F, Loingeville F, Dubar M. 2021. Antibiotic use in periodontal therapy among French dentists and factors which influence prescribing practices. Antibiotics (Basel) 10:303. 10.3390/antibiotics10030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, Liu C, Wang D, Zhang X. 2019. Intrinsic and external determinants of antibiotic prescribing: a multi-level path analysis of primary care prescriptions in Hubei, China. Antimicrob Resist Infect Control 8:132. 10.1186/s13756-019-0592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen R, Calder L, Thomas DW. 2000. Antibiotic prescribing for dental conditions: general medical practitioners and dentists compared. Br Dent J 188:398–400. 10.1038/sj.bdj.4800493. [DOI] [PubMed] [Google Scholar]
- 33.Roque F, Herdeiro MT, Soares S, Teixeira Rodrigues A, Breitenfeld L, Figueiras A. 2014. Educational interventions to improve prescription and dispensing of antibiotics: a systematic review. BMC Public Health 14:1276. 10.1186/1471-2458-14-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianco A, Licata F, Zucco R, Papadopoli R, Pavia M. 2020. Knowledge and practices regarding antibiotics use. Evol Med Public Health 2020:129–138. 10.1093/emph/eoaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianco A, Licata F, Trovato A, Napolitano F, Pavia M. 2021. Antibiotic-dispensing practice in community pharmacies: results of a cross-sectional study in Italy. Antimicrob Agents Chemother 65:e02729-20. 10.1128/AAC.02729-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elgreatly A, Kolker JL, Guzmán-Armstrong S, Qian F, Warren JJ. 2019. Management of initial carious lesions: Iowa survey. J Am Dent Assoc 150:755–765. 10.1016/j.adaj.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Park JH, Bay C. 2019. A survey of pediatric dentists on the treatment timing and modalities for white spot lesions in the United States. J Clin Pediatr Dent 43:27–33. 10.17796/1053-4625-43.1.6. [DOI] [PubMed] [Google Scholar]
- 38.Mayberry ME, Norrix E, Farrell C. 2017. MDA dentists and pregnant patients: a survey of attitudes and practice. J Mich Dent Assoc 99:54–62, 83. [PubMed] [Google Scholar]
- 39.Italian National Institute of Statistics. 2020. Fourth quarter 2019 export of Italian regions. Italian National Institute of Statistics, Rome, Italy. https://www.istat.it/it/files//2020/03/Export-of-Italian-regions-December-2019-2.pdf. Accessed 28 March 2021. [Google Scholar]
- 40.Hosmer DW, Lemeshow S. 2000. Applied logistic regression, 2nd ed. Wiley, New York, NY. [Google Scholar]
- 41.StataCorp LLC. 2019. Stata statistical software: release 16. StataCorp LLC, College Station, TX. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set was deposited in the Mendeley Data repository (https://doi.org/10.17632/gbvhzbjnsm.1).