Figure 3. Pharmacologic and Virologic Data for HIV Infections in the Cabotegravir Group.
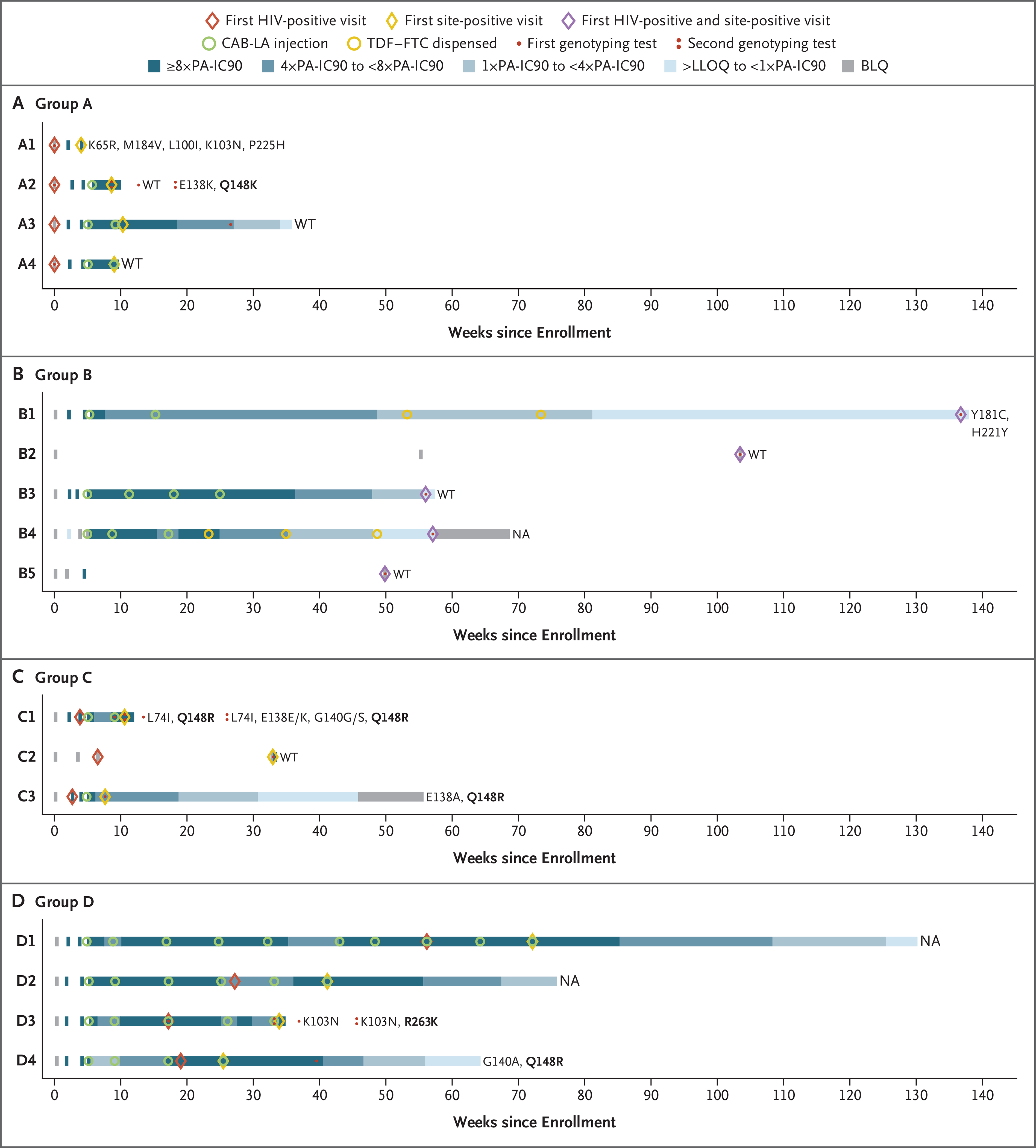
Panels A through D show the timing of key events for the 16 infections that occurred in the cabotegravir group. These infections were classified into four groups: group A (Panel A) includes infections that occurred before enrollment; group B (Panel B) includes infections that occurred with no recent exposure to cabotegravir; group C (Panel C) includes infections that occurred before cabotegravir injection; and group D (Panel D) includes infections that occurred in participants with appropriately timed CAB-LA doses and expected plasma cabotegravir concentrations. The “first HIV-positive visit” refers to the first visit at which the participant was determined to be HIV positive. The “first site-positive visit” refers to the first visit at which evidence of HIV infection was identified at the trial site. HIV genotyping results are shown to the right of each horizontal bar. Major resistance mutations are shown for nucleoside or nucleotide reverse-transcriptase inhibitors (K65R and M184V) and nonnucleoside reverse-transcriptase inhibitors (L100I, K103N, Y181C, H221Y, and P225H). All integrase strand-transfer inhibitor (INSTI) resistance mutations are shown (L74I, E138K or E138E/K, E138A, G140A, G140G/S, Q148R or Q148K, and R263K); major INSTI mutations are shown in bold text. Genotyping results are shown for the first visit at which the viral load was 500 copies or more per milliliter and for follow-up visits at which such a viral load occurred, denoted as one dot or two dots, respectively. The number of days between the first HIV-positive visit and the visit with additional mutations was 60 days for case A2, 10 days for case C1, and 112 days for case D3. NA indicates that the genotyping result was not available (either no visit occurred at which viremia was found to be sufficient for performance of the genotyping assay or the assay failed to produce a genotyping result). BLQ denotes below the limit of quantification, LLOQ lower limit of quantification, PA-IC90 protein-adjusted 90% cabotegravir inhib itory concentration, and WT wild type.
