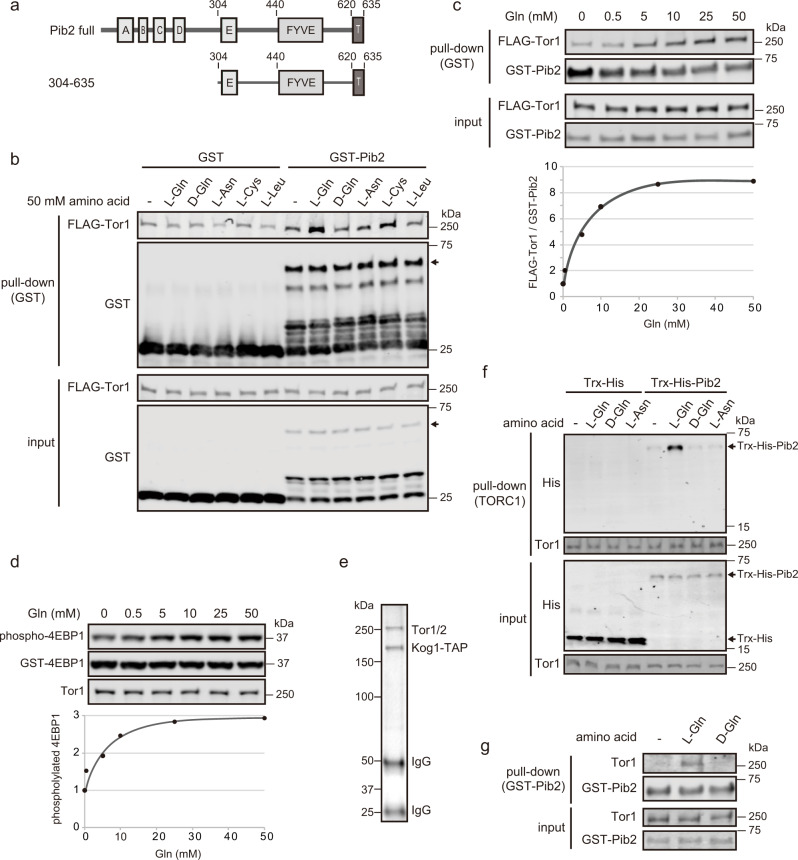
Fig. 1. In vitro reconstitution of the glutamine-responsive Pib2-TORC1 interaction.
a Schematic diagram of full-length Pib2 with defined motifs and the truncation mutant used in (b, c, f, and g). b L-glutamine and L-cysteine enhance the Pib2-TORC1 interaction in vitro. Lysates from FLAG-Tor1 expressing yeast cells (TS269) were subjected to pull-down assays with bacterially-expressed GST-Pib2(304-635) and the indicated amino acids (final concentration 50 mM). c The Pib2-TORC1 interaction depends on glutamine dose. Pull-down assays were performed as in (b), except that the indicated concentrations of L-glutamine were added to the cell lysates. The line graph shows GST-Pib2-bound FLAG-Tor1 normalized to sample without glutamine. d In vitro Pib2-dependent TORC1 activity depends on glutamine dose. In vitro TORC1 kinase assays monitored Pib2-dependent TORC1 activation using permeabilized yeast cells as the kinase source with the indicated concentrations of L-glutamine. The line graph shows the ratio of phosphorylated 4EBP1 to total 4EBP1 normalized to sample without glutamine. e Silver-stained SDS-PAGE gel for TORC1 purified from Kog1-TAP-expressing yeast cells (RL171-2a) using IgG-coupled magnetic beads. f Pib2 and TORC1 are sufficient to induce their glutamine-induced interaction. Bacterially-expressed and purified Trx-His-Pib2(304-635) and TORC1 purified from yeast on magnetic beads were incubated with the indicated amino acids (30 mM). Trx-His-Pib2(304-635) co-precipitation with TORC1 was detected by western blotting. g Induction of the Pib2-TORC1 interaction by glutamine is stereospecific. TORC1 was purified as in (e) and eluted from the beads using tobacco etch virus protease before being incubated with bacterially-expressed GST-Pib2(304-635) and the indicated amino acids (30 mM). TORC1 co-precipitation with GST-Pib2 was detected by western blotting.