Figure 2. Targets of chromatin modifiers altered in brain tumours.
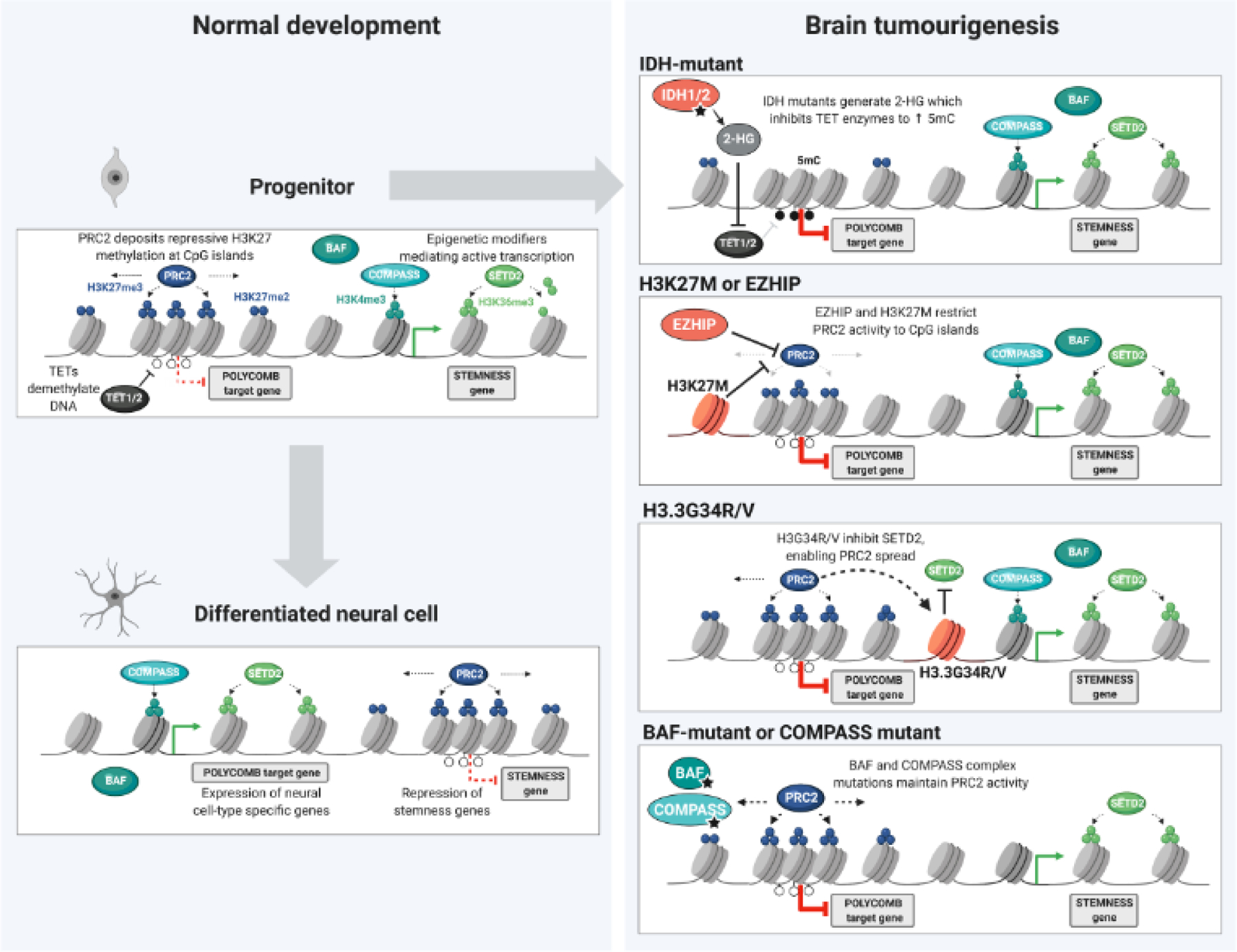
Appropriate differentiation of neural lineages requires remodeling of chromatin landscapes through the interplay of polycomb repressive complex 2 (PRC2), acting in opposition to BRG1/BRM associated factors (BAF) complexes and Complex Proteins associated with Set1 (COMPASS). Abnormal PRC2 occupancy at genomic loci involved in fate specification, and expression of stemness associated genes, can stall differentiation and promote tumorigenesis linked to enhanced self-renewal. In stem cells, polycomb targets are prone to acquisition of repressive 5 methyl-cytosine through the inhibition of histone and DNA demethylases. Restraining the spread of PRC2 through H3K27M or Enhancer of Zeste Homologs Inhibitory Protein (EZHIP) aberrant expression maintains repression of specific polycomb targets and an active chromatin at stemness-associated genes. Deposition of H3.3G34R/V mutant histone prevents SET Domain containing 2 (SETD2) deposition of H3K36me3, thereby facilitating PRC2 redistribution to genic regions. The loss of BAF and COMPASS functions further prevents the conversion from repressed PRC2 targets to an active state, and is associated with stalled neural differentiation.
