Figure 4. Manipulation of driver events determines reversibility of tumour epigenomes.
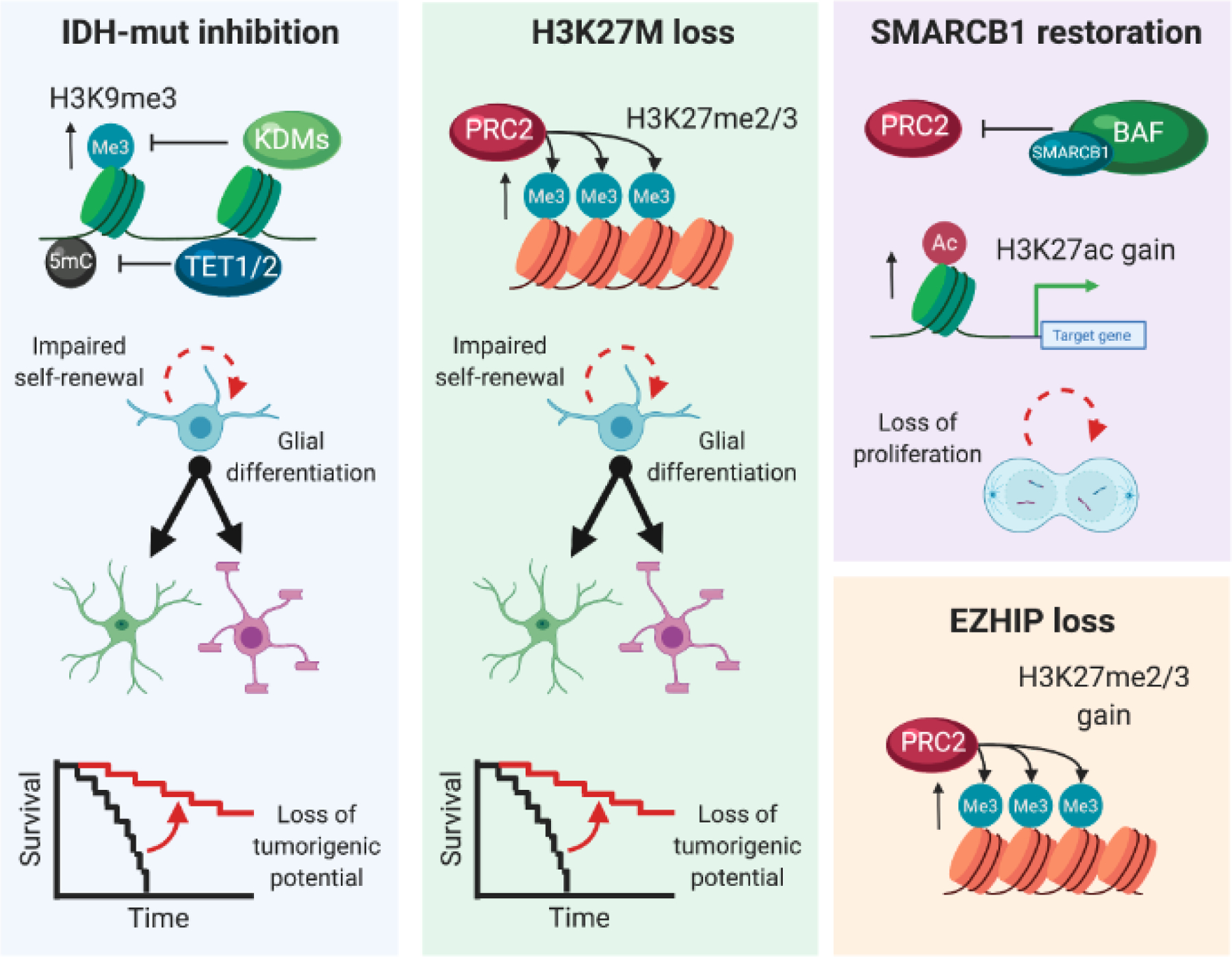
The manipulation of driver events remodeling the epigenome serves to characterize the reversibility of their effects, and the dependence of transformed cells on their function to proliferate, self-renew, and form tumours. The inhibition of 2-HG production by IDH1-R132H using a small molecule drug elevates 5-hydroxymethylcytosine, diminishes histone methylation and promotes glial differentiation in vitro, while delaying tumour burden in xenografts. The knockout or knockdown of the H3F3A-K27M mutant allele in tumour-derived cell lines restores PRC2 activity and H3K27me2/3deposition, renders the cells capable of enhanced glial differentiation and delays or abolishes the potential to form xenograft tumours. Similar trends are observed upon knockout of EZHIP, variably diminishing cell proliferation in some contexts. The restoration of SMARCB1 expression in ATRT lines reversed the loss of H3K27ac to reactivate silenced genes and decrease proliferation.
