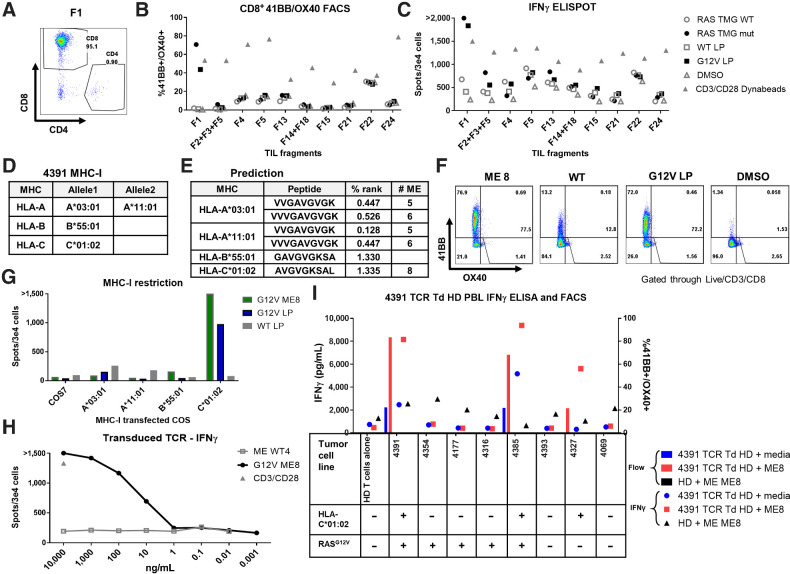
Figure 2.
TCR-recognized RASG12V hotspot mutation discovered by TIL screening. TIL fragments were screened for reactivity against autologous DCs transfected with RAS TMG (WT/Mutated) or loaded with 24-mer long peptide (RASG12V/RASWT). Coculture with DCs loaded with DMSO was used as a negative control. Anti-CD28/CD3 beads were used as a positive control. A, Most fragments, after gating on the live/CD3+ cells and including fragment 1 (F1), primarily contained CD8+ cells. Fragment reactivity tested in flow cytometry assays measured upregulation of 41BB+ and OX40+ (B) or by IFN-γ ELISpot (C). D, Table containing the patient 4391 MHC-I restriction elements. E, Table with the best-predicted RASG12V minimal epitopes (low % rank) to bind to the patient MHCs by the NetMHC 4.0. F, To determine which ME is recognized by TIL F1, cells were cocultured with autologous DC preloaded with RASG12V ME8, with LP (RASG12V/RASWT), or with DMSO at an equivalent concentration. Reactivity was determined by upregulation of 41BB and OX40 surface markers in live/CD3+/CD8+ by flow cytometry. G, For testing the MHC-I restriction element, TIL F1 were cocultured with COS7 cells pretransfected with patient's class I HLA DNA plasmids and loaded with LP (RASG12V/RASWT) or ME8. Reactivity was determined by IFNγ ELISpot. One TCR was identified from patient 4391 TIL fragment F1 after single-cell sequencing. This TCR was virally transduced to healthy donor (HD) PBLs. H, The TCR avidity was tested in the transduced cells by coculture with DCs loaded with different concentrations of RASG12V/RASWT 9-mer ME8 or the equivalent WT sequence WT4. The cells were tested via IFNγ ELISpot, with anti-CD28/CD3 beads used as a positive control. I, The TCR specificity and tumor recognition tested by coculturing the TCR-transduced HD cells with different TC lines with or without (±) RASG12V and HLA-C*01:02. TC lines were loaded with RASG12V ME8, or not peptide-loaded (media). Unloaded TC lines expressed their native peptidomes (including RASG12V). The reactivity was tested by IFNγ ELISA and by flow cytometry assay for 41BB/OX40 surface marker upregulation in live/CD3+/mTCR+/CD8+ cells. Untransduced HD loaded with ME8 was used as a negative control.