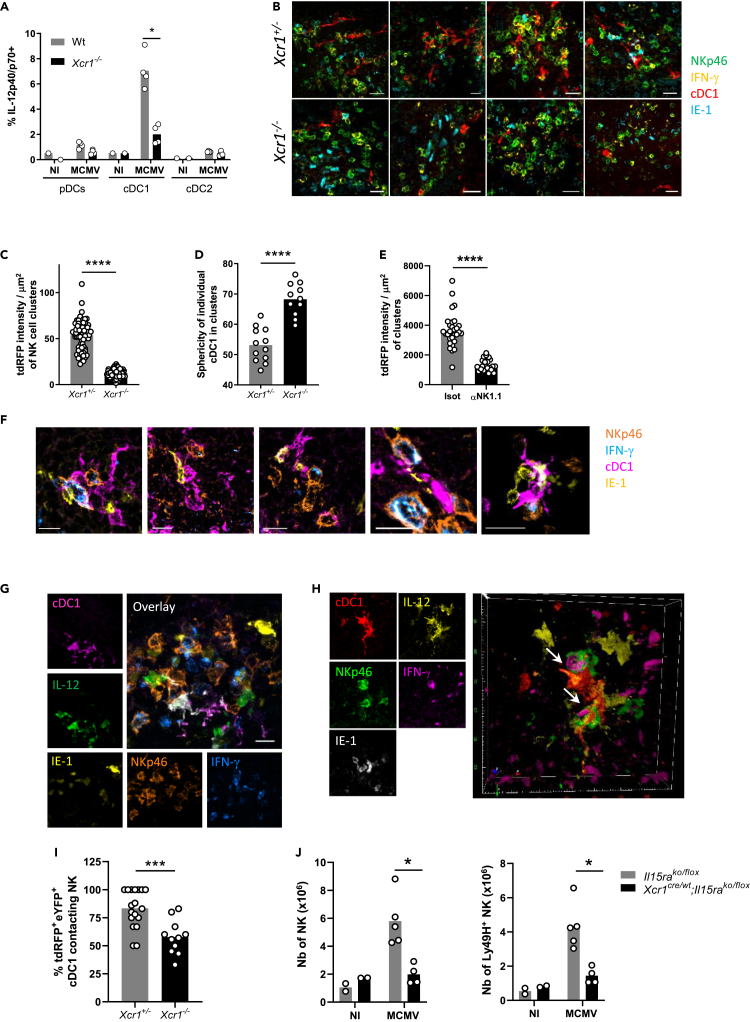
Figure 2.
XCR1 regulates cDC1 repositioning into NK cell clusters where IL-12-expressing cDC1 contact IFN-γ-producing NK cells
(A) Spleens were harvested at 40 h (A–I) or 4 days (J) after MCMV infection. (A) Analysis of IL-12p40/p70 production by splenic DCs of Xcr1−/− and Wt animals. One experiment representative of at least 4 independent ones with at least 4 mice per infected group is shown. NI, noninfected; n.s., nonsignificant; ∗, p < 0.05.
(B) Visualization of cDC1 in individual NK cell cluster in KarmaCre;Rosa26tdRFP;Xcr1−/− mice and their Xcr1+/− littermate controls. Spleen sections (scale bar: 20 μm) were stained for NKp46 (green), IFN-γ (yellow), tdRFP (cDC1, red), and IE-1 (cyan).
(C) Quantification of the dRFP intensity per NK cell cluster area (μm2) as a mean to evaluate cDC1 presence in individual NK cell cluster. For B and C, two independent experiments with at least 2-3 mice per group were pooled. An average of two clusters was analyzed in each mouse. ∗∗∗∗, p < 0.0001.
(D) Analysis of cDC1 sphericity when in NK cell clusters. We used the HK-means tool (Dufour et al., 2008) from Icy software to segment the tdRFP signal in each cluster, to computationally define and assess the sphericity of individual cDC1.
(E) Quantification of the dRFP intensity per μm2 as a mean to evaluate cDC1 relocalization in clusters in mice depleted of NK1.1+ cells upon MCMV infection. Clusters were defined as >10 IFN-γ+ cells around IE-1+ cells situated in the MZ.
(F) Visualization of tripartite interactions between cDC1, MCMV-infected cells (IE-1+) and activated IFN-γ-producing NK cells in marginal clusters. A representative image from 4 distinct MCMV-infected control spleens is shown (scale bar: 10 μm).
(G) Analysis of IL-12-producing cDC1 in NK cell clusters in KarmaCre;Rosa26tdRFP;Il12eYFP+ mice. The color obtained from overlaying green IL-12-eYFP+ on purple tdRFP-expressing cDC1 is white. Scale bar: 20 μm.
(H) Visualization of physical contacts between IL-12-eYFP-expressing cDC1 and IFN-γ-producing NK cells (arrows) in marginal ring clusters. The micrographs shown (G and H) are representative of the analyses of 6 mice from two independent experiments.
(I) Proportion of tdRFP+ eYFP+ cDC1-contacting NK cells within individual marginal zone cluster in KarmaCre;Rosa26tdRFP;Il12eYFP+;Xcr1−/− mice and littermate controls. Two independent experiments with 2-3 mice per infected group were pooled. An average of 3.5 and 2.2 clusters was analyzed in Xcr1+/− and Xcr1−/−KarmaCre;Rosa26tdRFP;Il12eYFP+ mice, respectively. ∗∗∗, p < 0.001.
(J) Analysis of NK cell absolute number 4 days after infection in the spleen of Xcr1Cre/wt;Il15raKO/flox mice and their respective controls (Il15raKO/flox). One experiment representative of at least 2 independent ones with at least 4 mice per infected group is shown. NI, noninfected; n.s., nonsignificant; ∗, p < 0.05.
See also Figure S2.