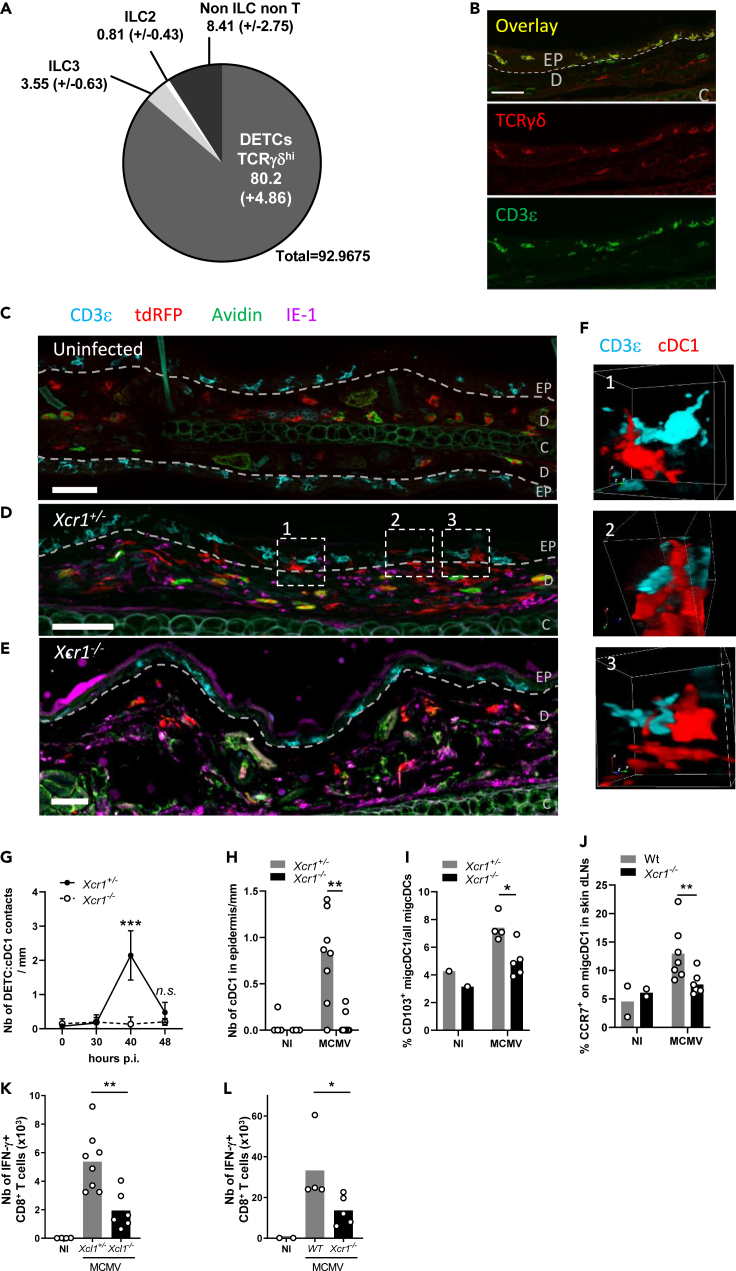
Figure 7.
Dermal cDC1 make cell/cell contacts with Xcl1-expressing DETCs
(A) Analysis of the lymphocyte heterogeneity within mTFP1+ cells in the skin of Xcl1mTfp1fl/fl mice at steady state. One representative experiment of 2 independent ones with at least three mice per group is shown.
(B) DETC staining in the skin at steady state. DETCs are CD3ε+TCRγδ+ lymphocytes localized in the epidermis. The dotted line delineates the basal layer separating the dermis (D) from the epidermis (EP). C, cartilage. Scale bar: 50 μm
(C–E) Analysis of cDC1 localization and contacts with DETCs in the skin, at steady state in KarmaCre;Rosa26tdRFP;Xcr1+/− mice (C), and at 40 h after MCMV infection in KarmaCre;Rosa26tdRFP;Xcr1+/− (D) and KarmaCre;Rosa26tdRFP;Xcr1−/− (E) mice. The dotted line delineates the basal layer separating the dermis from the epidermis. Skin sections (scale bar: 50 μm) were stained for tdRFP (red; cDC1), CD3ε (cyan), TCRγδ (yellow), IE-1 (purple), and Avidin (green; mast cell). EP, epidermis; D, dermis; C, cartilage.
(F) 3D visualization of DETC/cDC1 contacts identified in (D).
(G) Kinetics of cDC1/DETC contacts occurring in the epidermis and through the epidermis-dermis border per mm of skin length, during MCMV infection. Data are represented as mean (+/− SEM). p.i., postinfection.
(H) Quantification of cDC1 nuclear bodies inside the epidermis per mm of skin length 40 h after skin infection. For (G-H), two independent experiments with at least 4 mice per infected group were pooled. n.s., nonsignificant; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
(I) Proportion of CD103+ migcDCs in the ear-draining LN of Xcr1−/− and littermate controls 48 h after skin infection.
(J) Analysis of CCR7 expression on migcDCs in ear-draining LN 48 h after skin infection of Xcr1−/− and Wt mice. Two independent experiments with at least 3 mice per infected group were pooled. ∗∗, p < 0.01.
(K and L) Analysis of m45-specific CD8+ T cell response in Xcl1−/− mice (K), Xcr1−/− mice (L) and their respective controls 6 days after MCMV infection of the ear. CD8+ T cells from ear-draining LN were stimulated in vitro for 4 h with m45 peptide. Two independent experiments with at least 3 mice per infected group were pooled for (K), and one experiment with at least 4 mice per group is shown for (L). ∗, p < 0.05∗∗, p < 0.01.
See also Figure S7.