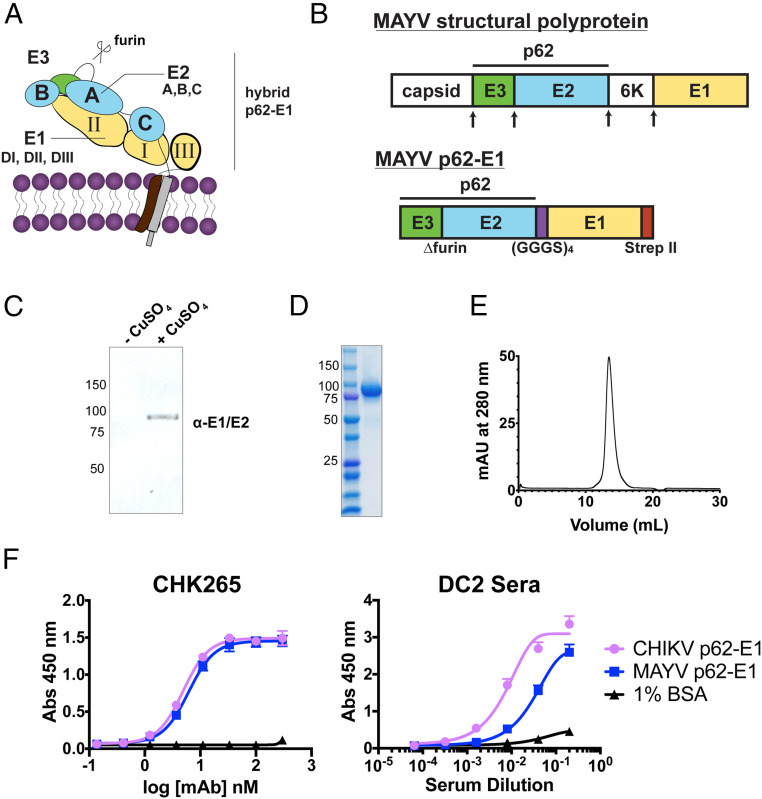
Fig. 1.
Design, expression, and purification of MAYV p62-E1 heterodimer for single–B cell sorting. (A) Structural organization of the alphavirus glycoprotein. (B) Schematic of MAYV structural protein and p62-E1 construct. Arrows indicate the sites of proteolytic cleavage. MAYV p62-E1 contains a mutated furin cleavage site, a glycine-serine linker connecting p62 to E1, and a C-terminal strep II tag for purification. Immunoblot (C) and SDS-PAGE (D) of MAYV p62-E1 generated in S2 cells. MAYV p62-E1 was detected by an anti-E1/E2 antibody. (E) Size exclusion chromatogram of MAYV p62-E1. Sample was run on Superdex S200 10/300 AKTA column. A retention time of 13.5 mL was observed, consistent with molecular weight of ∼100 kDa. (F) ELISA reactivity of CHK-265 toward CHIKV/MAYV p62-E1 (Left) and serological ELISA (Right) of patient DC2 serum toward CHIKV/MAYV p62-E1. ELISAs were performed twice independently in triplicate wells (mean ± SD).