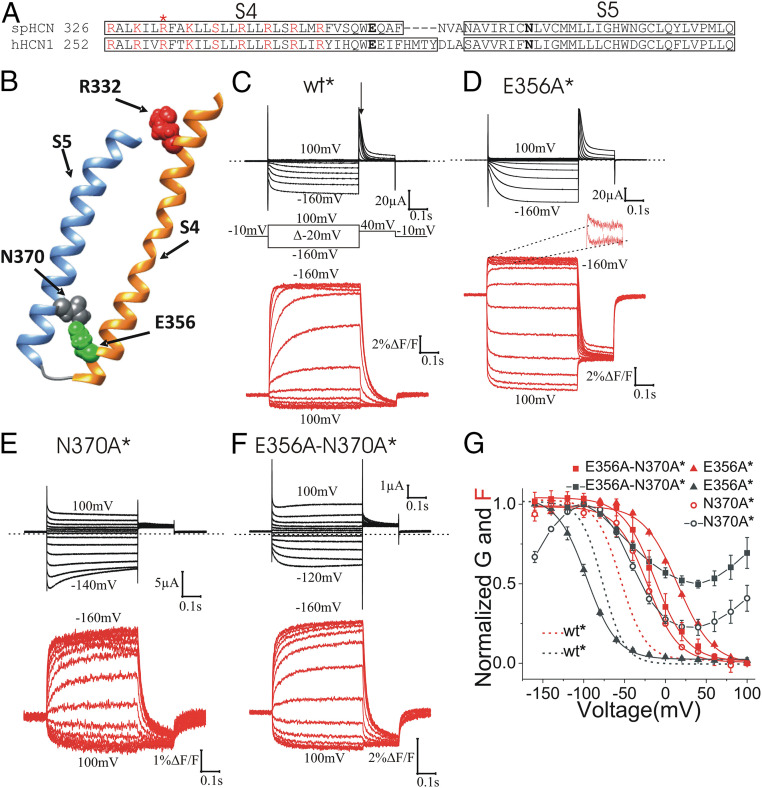
Fig. 1.
E356 and N370 stabilize S4 in the outward state. (A) Amino acid sequence alignment of S4 and S5 segments of spHCN and hHCN1 channels. Box indicates the extend of the α-helical regions of S4 and S5. The asterisk (*) indicates R332, which was mutated to cysteine for fluorescence labeling. Positively charged residues in S4 are marked in red. E356 and N370 are marked in bold. (B) spHCN homology model showing the interaction of E356 and N370 in closed channels with S4 outward. (C–F) Representative current (black) and fluorescence (red) from (C) wt*, (D) E356A* (second component zoomed out in Inset), (E) N370A*, and (F) E356A-N370A* channels. Arrow in C marks where tail currents were measured for G(V)s. (G) Normalized G(V) and F(V) curves of wt*, E356A*, N370A*, and E356A-N370A* channels. Mean ± SEM. In a previous publication we showed that a polar residue is needed at position 370 to close completely HCN channels at positive voltages (28).