Abstract
Secreted phospholipases A2 (sPLA2s) form a widespread group of structurally-related enzymes that catalyse the hydrolysis of the sn-2 ester bond of glycerophospholipids to produce free fatty acids and lysophospholipids. In humans, nine catalytically active and two inactive sPLA2 proteins have been identified. These enzymes play diverse biological roles, including host defence against bacteria, parasites and viruses. Several of these endogenous sPLA2s may play a defensive role in viral infections, as they display in vitro antiviral activity by both direct and indirect mechanisms. However, endogenous sPLA2s may also exert an offensive and negative role, dampening the antiviral response or promoting inflammation in animal models of viral infection. Similarly, several exogenous sPLA2s, most of them from snake venoms and other animal venoms, possess in vitro antiviral activities. Thus, both endogenous and exogenous sPLA2s may be exploited for the development of new antiviral substances or as therapeutic targets for antagonistic drugs that may promote a more robust antiviral response. In this review, the antiviral versus proviral role of both endogenous and exogenous sPLA2s against various viruses including coronaviruses is presented. Based on the highlighted developments in this area of research, possible directions of future investigation are envisaged. One of them is also a possibility of exploiting sPLA2s as biological markers of the severity of the Covid-19 pandemic caused by SARS-CoV-2 infection.
Keywords: Secreted phospholipase A2, Antiviral action, Host defence, Viruses, SARS-CoV-2, Covid-19
1. Introduction
Phospholipases A2 (PLA2s) comprise a superfamily of intracellular and secreted enzymes that catalyse the hydrolysis of glycerophospholipids at the sn-2 position, releasing free fatty acids and lysophospholipids [1,2]. Secreted PLA2s (sPLA2s) completely differ from intracellular PLA2 enzymes in structure, enzymatic properties, tissue distribution and biological functions. sPLA2s constitute a family of structurally-related enzymes which are widespread in nature and exert highly diverse biological functions [[3], [4], [5], [6], [7]]. Nine catalytically-active sPLA2s and two catalytically-inactive sPLA2-like proteins (classified into groups and subgroups) are present in humans and other mammals [3]. These human sPLA2s are small proteins of 14–18 kDa with 6–8 disulphide bonds and a highly conserved His–Asp catalytic dyad at the active site using Ca2+ as a key cofactor for catalysis. They are also termed endogenous sPLA2s and are involved in a myriad of physiological and pathological processes, from digestion, lipid metabolism, skin homeostasis, spermatogenesis or host defence to regulation of the immune response and inflammation in disease conditions such as asthma, sepsis and cardiovascular diseases. sPLA2s participate to such processes by various mechanisms, for instance by modifying the lipid composition of cellular membranes or by producing pro- and/or anti-inflammatory lipids from both cellular and noncellular lipid components such as cell membranes, lipoproteins, microvesicles, mitochondria or foreign phospholipids from microbes and food [3,8,9].
sPLA2s are also found in large amount in animal venoms, especially in those from snakes, scorpions and bees [[5], [6], [7]]. Venom sPLA2s are structurally similar to endogenous mammalian sPLA2s but display toxic effects including neurotoxicity, myotoxicity, anticoagulant activity, cardiotoxicity and haemolytic activity. They can be considered as exogenous sPLA2s when injected to mammals, acting as weapons to kill preys. However, they are also viewed as pharmacological agents to possibly cure diseases [[10], [11], [12]]. As such, they are valuable research tools for analysing physiological and pathophysiological conditions in humans as well as promising molecules for the development of new therapeutic agents [[13], [14], [15], [16]].
Both endogenous and exogenous sPLA2s play direct and indirect roles in host defence against various types of bacteria, parasites and viruses [[17], [18], [19], [20], [21]]. For example, an early study showed that several snake and bee venom sPLA2s exhibit direct antiviral activity by blocking HIV-1 virus entry into host cells [21]. Later, it was shown that group III, V and X (GIII, GV and GX) endogenous sPLA2s play antiviral roles against adenoviruses and HIV-1 by different mechanisms [[22], [23], [24]]. Other studies have shown that the levels of endogenous sPLA2s are upregulated after infection by dengue virus, H1N1 or coronavirus [[25], [26], [27], [28]] and can modulate the antiviral response by indirect immune mechanisms [27,28].
In this review, we present the different antiviral activities of both endogenous and exogenous sPLA2s but also their antagonistic role in worsening viral infection. It appears that sPLA2s interfere with the life cycle of various viruses at different steps and also act indirectly by immunomodulation of the antiviral immune response. Some of their modes of antiviral action may be used to formulate strategies for the development of diagnostic and therapeutic procedures, perhaps even agents to treat viral infections.
2. Human sPLA2s
In mammals, mainly based on information obtained from mice (m) and humans (h), 11 to 12 sPLA2 members have been identified: GIB, GIIA, GIIC (pseudogene in humans), GIID, GIIE, GIIF, GIII, GV, GX, GXIIA, GXIIB and otoconin-90. These sPLA2s are usually classified into three structural collections, i.e. the collection of I/II/V/X sPLA2s, and the collections of III and XII sPLA2s [3,4,29]. The corresponding human sPLA2 gene names are PLA2G1B, PLA2G2A, PLA2G2C, PLA2G2D, PLA2G2E, PLA2G2F, PLA2G3, PLA2G5, PLA2G10, PLA2G12A, PLA2G12B and OC90.
Mammalian sPLA2s are differentially expressed in tissues and cell types, and play distinct, mostly non-redundant, roles that may be dependent or independent of their enzymatic activity. All but two mammalian sPLA2s are enzymatically active, with a wide range of specific activities depending on the nature of glycerophospholipids, and in vitro versus in vivo settings [30,31]. GXIIB sPLA2 is a catalytically-inactive sPLA2-like protein due to substitution of the highly conserved histidine by a leucine residue in the active site [29]. Despite its obvious lack of enzymatic activity, the protein has been shown to play a role in hepatic triglyceride metabolism by an unknown molecular mechanism [32]. Otoconin-90 is another sPLA2-like protein found in the inner ear and involved in balance regulation [[33], [34], [35]]. It possesses a tandem repeat of two sPLA2-like domains [33] with substitutions in key amino acids involved in enzymatic activity, rendering it catalytically inactive. It can however still bind Ca2+ ion, which is important for its binding properties within the inner ear extracellular matrix called otoconia [[33], [34], [35]]. The proposed biological functions of endogenous sPLA2s are largely based on observations from transgenic and/or knock-out mouse lines [8,36,37]. Although a large number of important sPLA2 functions have been revealed by using this approach, it should be emphasized that the results obtained in mice and humans are not always comparable. For example, GIIC sPLA2, which is abundantly expressed in meiotic cells in the mouse testis, is found as a pseudogene in the human genome [38,39]. Similarly, GIIE sPLA2 may play various roles related to inflammation and obesity in the mouse [40] but is barely expressed in most human cells [41,42]. GX sPLA2 is present in the acrosome of mouse spermatozoa while it seems that GIID sPLA2 and/or other sPLA2s are present in human spermatozoa [[43], [44], [45]]. Furthermore, most studies were performed with sPLA2 knock-out mouse lines in the C57Bl/6 background, where the GIIA sPLA2 is naturally absent due to a frameshift mutation [46], while this sPLA2 is highly expressed in other mouse strains like Balb/C [46] or in human tissues where it participates to inflammation and associated diseases, cancer and antibacterial defence among other functions [3,[47], [48], [49]]. This raises issues for possible redundancy, overlooked or overestimated roles of certain sPLA2s, and the translational impact of the findings from mice to humans. Therefore, with the aim to better understand the biological roles of the different human sPLA2s in diseases, which may be useful for diagnosis and treatment, it is reasonable to combine the key observations obtained from mouse models with those directly derived from human studies, from tissue distribution and function of human sPLA2s in healthy and disease conditions up to analyses of sPLA2 gene polymorphisms and mutations [[50], [51], [52], [53], [54], [55], [56]].
Finally, it should be mentioned that endogenous sPLA2s exhibit complex enzymatic properties depending on the lipid membranes that they will encounter once secreted. Indeed, they can act on a large variety of lipid substrates to produce multiple lipid mediators or modify the biochemical or biophysical properties of cell membranes or extracellular microvesicles, making their action both complex and unique to the microenvironment in which each of the different sPLA2s operate. sPLA2s act primarily on different extracellular or noncellular phospholipids such as those of the plasma membrane, lipoproteins, lipid microparticles, lung surfactant, skin lipid bodies, food and bacterial membranes, mitochondria, but also on intracellular membranes during their secretion [8,19,30,31,50], [[57], [58], [59], [60], [61], [62], [63]]. Products of sPLA2 phospholipolysis, free fatty acids and lysophospholipids, are involved in a number of cellular pathways by generation of multiple bioactive lipid mediators, promotion of membrane remodelling, modification of extracellular noncellular lipid components, such as microparticles and lipoproteins, lung surfactant, or degradation of foreign phospholipids from microbes, microbiota and dietary components in response to given microenvironmental cues.
Some of the major biological effects of endogenous sPLA2s and their potential involvement in various human disorders are listed in Table 1 .
Table 1.
Key biological roles of endogenous mammalian sPLA2s, including their potential antiviral and/or proviral biological roles (underlined). The table is largely summarized from Refs. [9,40]; with additional information on GIB [56,64], GIII [23], GV [22,23], GX [22,23,27], GXIIA [[65], [66], [67], [68]] and otoconin-90 [[33], [34], [35]].
| Endogenous mammalian sPLA2 | Tissue and cell distribution | Physiological role | Pathophysiological role |
|---|---|---|---|
| GIB | pancreatic acinar cells, duodenum, ileum, stomach, lung, blood, spermatozoa | digestion of dietary phospholipids in the small intestine, immune response | obesity, lung inflammation, anergy of T cells in HIV infection (i.e.proviral role), anthelmintic |
| GIIA | intestine, Paneth cells, platelets, leukocytes, synoviocytes, skin, biological fluids (blood, tears, seminal and synovial fluid, lung alveolar space) | regulation of intestinal microflora, inflammation and lipid metabolism, role in host defence and in innate and immune responses, direct killing of Gram-positive bacteria and of commensal bacteria | amplification of inflammation in many inflammatory diseases and cardiovascular diseases, role in sepsis and various bacterial and viral infections, role in cancer and metastasis |
| GIID | immune system, lymphoid and dendritic cells, M2 macrophages, regulatory T cells | anti-inflammatory, resolution of Th1 immunity, candidate as an immune checkpoint | decrease of immune response, anti-tumour response, antiviral immunity in H1N1 and SARS-CoV infections(i.e.proviral role) |
| GIIE | adipocytes, hair follicles, uterus | fat deposition in adipose tissue and liver, hair follicle homeostasis, lipid metabolism | obesity (age-induced anti-obesity?), skin disorders |
| GIIF | skin, suprabasal keratinocytes | skin homeostasis, lipid metabolism | skin disorders such as cancer and psoriasis |
| GIII | intestine, liver, epididymal epithelial cells, mast cells, colonic epithelial cells, aorta, skin, brain | male fertility, mast cell maturation | anaphylaxis, colon cancer and colitis, atherosclerosis, skin inflammation, antiviral (adenovirus) role |
| GV | bronchial epithelial cells, endothelial cells, macrophages, dendritic cells, hematopoietic cells, cardiomyocytes, aorta, hypertrophic adipocytes, pancreatic β-cells, gut epithelium, brain | host defence, surfactant degradation, M2 macrophage polarization, Th2 immunity, phagocytosis of microorganisms, phagocytosis of immune complexes, apoptosis of injured myocardial cells, reduced adipose tissue inflammation by unsaturated fatty acids, anti-arthritis, anti-obesity | airway lung injury, asthma, atherosclerosis, aortic inflammation, myocardial infarction, aneurysm, antiviral (adenovirus) role |
| GX | airway epithelium, intestinal mucosa, stomach, immune system, macrophages, adrenal glands, dorsal root ganglia, hematopoietic cells, neutrophils, adipocytes, sperm acrosome, hair follicles | enhanced lipid accumulation and TLR4 signalling in macrophages, reduced corticosteroid synthesis by downregulating adrenal steroidogenic acute regulatory protein, neuritogenesis and pain transmission, macrophage function, reduced Th1 immunity and atherosclerotic plaque formation, suppression of insulin secretion, phospholipid digestion in the gastrointestinal lumen, repression of adipogenesis by inhibiting liver X receptor activation, anti-atherosclerosis, anti-obesity, sperm fertility, hair homeostasis | airway inflammation, asthma, decreased tissue damage by neutrophils, hypercorticosteronemia, pain, aneurysm, myocardial infarction, diabetes, adiposity, alopecia, antiviral (adenovirus) role, antiviral immunity in H1N1 infection (i.e.proviral role) |
| GXIIA | ubiquitous, neurons, epithelial cells of gastrointestinal tract | neurogenesis and cognition, host defence, killing of Gram-positive bacteria in vitro, poorly understood molecular mechanisms | lipid metabolism disorders? |
| GXIIB | liver, intestine | triglyceride metabolism by unknown molecular mechanisms, anti-steatosis effect | lipid metabolism disorders? |
| Otoconin-90 | inner ear | maintenance of inner ear balance system | imbalance |
3. Antiviral activity of endogenous mammalian sPLA2s
Mammalian sPLA2s, especially GIIA sPLA2, are involved in direct and indirect host defence against various pathogens, including bacteria, parasites and viruses, and as such represent an important component of the innate immune system [17,18,24,27,28,47,56,64], [[69], [70], [71], [72]]. For example, studies as early as back in 1979 have shown that GIIA sPLA2 can exert direct killing of Gram-positive bacteria [73,74]. It was a result of the ability of this enzyme, due to its highly positive net charge, to penetrate the bacterial peptidoglycan envelope, to reach the bacterial plasma membrane and to hydrolyse it [18,47]. In addition, the same sPLA2 is responsible for an indirect host response triggering innate immune response, possibly via hydrolysis of lipid microvesicles or mitochondria released by activated platelets (or other cells) to release potent lipid mediators, or when certain bacterial strains manipulate the host defence activity within a bacterial niche [57,61,75]. Multiple studies have shown that GIIA sPLA2 has in vitro and in vivo antibacterial activity against various bacterial strains [17,18,47,69,70].
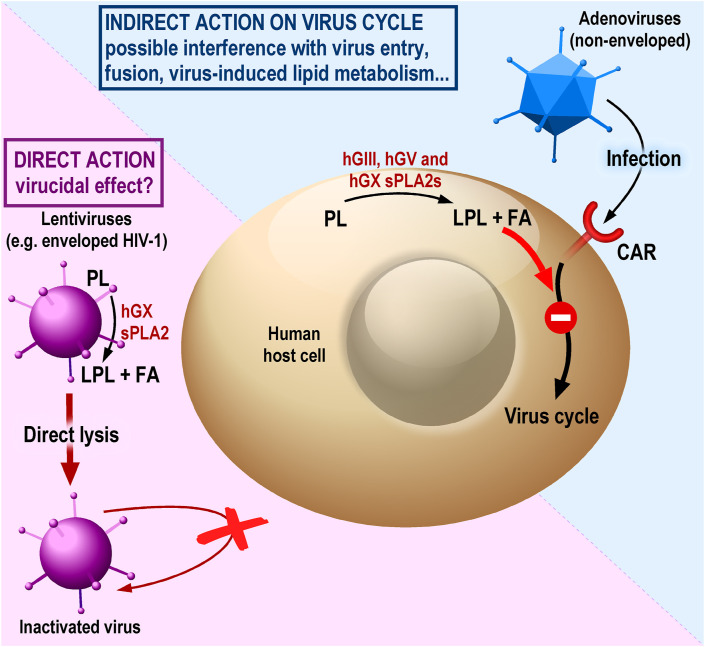
The antiviral activity of endogenous mammalian sPLA2s is much less documented than their antibacterial roles. It was first reported in vitro for two potent plasma membrane-hydrolysing sPLA2s, hGV and hGX sPLA2s, which could prevent host cells from being infected with adenoviruses [22,23] (Table 1). These two sPLA2s are expressed in human airway epithelium and macrophages, and the expression of hGV sPLA2 is upregulated by virus-related stimuli of these cells. Adenovirus particles were insensitive to exogenously added hGV and hGX sPLA2s. However, addition of recombinant hGV and hGX sPLA2s, but not hGIIA, to HEK293 cells suppressed the number and size of adenovirus plaque formation. In contrast to the main antibacterial action of sPLA2s, which depends on their direct hydrolytic action on bacterial membranes [70], the antiviral action of GV and GX sPLA2s was indirect and dependent on their ability to act on host cell membranes (Fig. 1 ), leading to a decrease in adenovirus entry by clathrin-mediated endocytosis. The observation that suppression of adenoviral infection by hGV and hGX sPLA2s depends on catalytic activity was confirmed with catalytically-inactive sPLA2 mutants and supported by the finding that lysophosphatidylcholine, an sPLA2-hydrolytic product of phosphatidylcholine enriched in the outer leaflet of the plasma membrane, also suppresses adenoviral infection. It was also suggested that the innate immune action of these sPLA2s may be directed not only against adenoviruses but also against other respiratory viruses [22]. Besides endogenous GV and GX sPLA2s, hGIII sPLA2 was also able to inhibit adenovirus entry into host cells [23]. It was shown that, in addition to the catalytically-active sPLA2 domain, the N-terminal domain unique to this enzyme is required for the anti-adenoviral effect observed in human bronchial epithelial cells. This suggests a slightly different antiviral mechanism in the case of hGIII sPLA2. By lipidomics analysis, the authors provided evidence that hGIII, hGV and hGX sPLA2s target different phospholipids in host cell membranes, all leading to production of lysophosphatidylcholine, which was proposed to be responsible for the antiviral action. However, the in vivo role of these sPLA2s against adenovirus infection remains to be demonstrated.
Fig. 1.
The antiviral action of endogenous sPLA2s in in vitro cell systems. Some sPLA2s, for example human, may act on viruses either directly, by degrading their lipid envelope (virucidal effect?), such as in the case of HIV-1, or indirectly, by acting on cell lipid membranes to prevent effective production of viral particles, such as in the case of non-enveloped adenoviruses. Via enzymatic activity, sPLA2s hydrolyse phospholipids (PL) from the virus or infected cellular membranes to produce lysophospholipids (LPL) and free fatty acids (FA) that can disturb some key steps of the viral life cycle or lipid metabolism of the host cell. The enveloped virus (e.g. HIV-1), with a lipid membrane derived from host plasma membrane, and the non-enveloped (no lipid) virus (e.g. adenovirus) are shown in violet and blue, respectively. The dashed line separates direct versus indirect sPLA2 actions on cells. CAR, coxsackievirus and adenovirus receptor.
In a subsequent study, it was found that endogenous hGX sPLA2 displays antiviral activity against different enveloped lentiviruses including HIV-1, while the enzyme was found unable to prevent adenovirus infection, which contrasts with the above study [24]. The enzymatic activity of hGX sPLA2 was necessary for the observed antiviral effect (Fig. 1). Other endogenous sPLA2s such as hGIIA, hGIID, hGIII, hGV and hGXIIA appeared to be ineffective, yet this was not analysed using pure recombinant enzymes. The antiviral activity was proposed to be due to a direct virucidal effect, i.e. direct hydrolysis and degradation of the viral membrane [24]. It was assumed that the lipid envelope of HIV-1 may be susceptible to the enzymatic action of hGX sPLA2, since it originates from the host cell membranes and is rich in its outer leaflet in zwitterionic phospholipids including phosphatidylcholine [76], while hGX sPLA2 has been shown to hydrolyse phosphatidylcholine from the plasma membrane of live cells [77,78]. However, more detailed lipidomics studies have shown that the lipid composition of the HIV virus particles is unique and distinct from the cell plasma membrane [[79], [80], [81]], leaving unknown the enzymatic properties of this sPLA2 on the surface of HIV particles and whether it has a real capacity to directly hydrolyse and kill the virus (i.e. via a virucidal effect). Furthermore, the concentration of recombinant hGX sPLA2 that inhibits HIV-1 infection in cellular assays versus the one that would produce the direct virucidal activity were not determined. Interestingly, the antiviral activity was observed despite the resistance of virus preparations to lysis by antibody-mediated complement activation, indicating that the sPLA2 may act independently of the adaptive immune response or when the complement pathway is ineffective. As hGX sPLA2 is highly expressed in the intestinal mucosa [82,83], the primary site of HIV-1 replication on natural infection, the authors suggested further studies to explore the role of hGX sPLA2 in the innate immunity against HIV-1 infection in the gastrointestinal system. Application of the recombinant protein and/or upregulation of hGX sPLA2 expression may thus limit viral replication and reduce the incidence of productive replication at the primary infection sites. However, as above for other sPLA2s, the in vivo role of hGX sPLA2 against HIV-1 infection remains to be demonstrated.
In a mouse model of infection by H1N1 influenza virus, mGX sPLA2 (Pla2g10 gene) has been involved in host antiviral response, but in a negative and likely indirect manner [27]. Survival after infection was greater in Pla2g10-deficient mice than in wild-type littermates. mGX sPLA2 was induced by H1N1 infection in bronchial epithelial cells and inflammatory cells, and was involved in the production of potent proinflammatory lipid mediators, likely contributing to acute lung injury. On the other hand, B and T cell antiviral responses were stronger in Pla2g10-deficient mice, suggesting that Pla2g10 promotes excessive lung inflammation and inhibits an efficient adaptive immune response, leading to poor survival. Collectively, these in vivo findings suggest that GX sPLA2 is a “proviral sPLA2” which contrasts with its above in vitro antiviral activity (Table 1). Based on data in this mouse model, GX sPLA2 may be a potential therapeutic target that should be inhibited during influenza infection. However, its in vivo antiviral or proviral role after infection with adenovirus or HIV-1 (with mouse adapted strains and related lentiviruses) remains unknown and should be tested in relevant animal models.
Soon after, it has been shown that mGIID sPLA2 (Pla2g2d gene) is negatively involved in the adaptive immune response in a mouse model of severe acute respiratory syndrome coronavirus (SARS-CoV) infection [28]. mGIID sPLA2 is a basic protein which was cloned from spleen and other lymphoid tissues and show a modest in vitro enzymatic activity as compared to its close paralog GIIA sPLA2 [30,84]. However, in vivo, mGIID sPLA2 was shown to preferentially cleave phosphatidylethanolamine esterified with various polyunsaturated fatty acids at the sn-2 position [85], releasing not only omega-6 arachidonic acid but also omega-3 eicosapentaenoic and docosahexaenoic acids, which are precursors of potent anti-inflammatory lipid mediators (resolvins) involved in resolution of inflammation during infection [86]. In mice and humans, GIID sPLA2 is expressed mainly in dendritic cells and M2 macrophages of secondary lymphoid organs, such as spleen and lymph nodes, is downregulated by pro-inflammatory stimuli, and is likely playing an anti-inflammatory and pro-resolving role [40]. For these reasons, it was termed a “resolving sPLA2” that ameliorates inflammation through mobilisation of pro-resolving lipid mediators [40,85]. A dual role for GIID sPLA2 as an immunosuppressive endogenous sPLA2 in inflammation and cancer was proposed in which it exerts an anti-inflammatory effect but decreases the anti-tumour effect, thereby promoting tumour development [87]. An immunosuppressive function of GIID sPLA2 was proposed even earlier, when it was cloned from lymphoid tissues of lymphotoxin-deficient mice [88], and shown to be highly expressed in regulatory T cells and to mediate the immunosuppressive function of these cells [89]. Regarding its role during viral infection, a critical role of mGIID sPLA2 in age-related susceptibility to SARS-CoV infection was observed [28], revealing a proviral role of this sPLA2. The authors carried out most of the experiments in mice, but the conclusions they made may be applicable to humans. In agreement with this, oxidative stress was found to induce GIID sPLA2 expression in mice and in human monocyte-derived macrophages. The study showed that GIID sPLA2, whose expression in lung dendritic cells increased with age in response to oxidative stress and also during coronavirus infection, contributed to worse outcome in mice infected with SARS-CoV. These results were supported by the observation that GIID sPLA2-deficient mice, in comparison with wild-type mice, showed a much higher rate of survival after infection with not only SARS-CoV but also influenza A viruses. On the contrary, the attenuated antiviral immunity in transgenic mice overexpressing GIID sPLA2 leads to severe lung inflammation and early death. The proposed molecular mechanism is that GIID sPLA2, expressed in lung dendritic cells, produces prostaglandin D2 and other lipid mediators that prevent the migration of dendritic cells to lymph nodes, thereby suppressing T-cell activation. This results in a decreased antiviral immunity and, hence, increased viral infection [90]. Accordingly, the specific inhibition of GIID sPLA2 represents a potentially therapeutic approach for the treatment of patients with severe respiratory infection due to influenza viruses or coronaviruses [40]. Very recently, it has been reported on the unexpected role of mGIID sPLA2 in the lung, serving as an important modulator of respiratory dendritic cell activation, with protective and pathogenic effects in respiratory CoV infections and immunization [91]. Together, these findings on a proviral role of GIID sPLA2 during infection with coronavirus and influenza viruses are similar to those observed for GX sPLA2 and H1N1 infection, suggesting that both sPLA2s may share some common molecular mechanisms of action and may be both targeted by specific inhibitors for the treatment of infections with respiratory viruses.
Finally, recent studies suggest that hGIB sPLA2 may negatively contribute to infection by HIV-1 by an indirect mechanism leading to anergy of CD4+ T cells, which may be at the basis of the chronic immunodeficiency syndrome observed in HIV patients [56]. This sPLA2 may act catalytically at the surface of CD4+ T cells in synergy with enzymatic cofactors such as the HIV gp41 envelope protein or degradation products. Importantly, the in vitro effects of the sPLA2 on CD4+ T cell anergy is dependent on its enzymatic activity and can be blocked by a specific neutralizing antibody, which may represent a therapeutic molecule. However, the effect of GIB sPLA2 on T cell anergy should be further demonstrated in in vivo models of HIV infection or T cell immune dysfunction. The cellular source of hGIB sPLA2 that is found in the plasma of HIV patients also remains to be determined.
4. Antiviral activity of exogenous sPLA2s
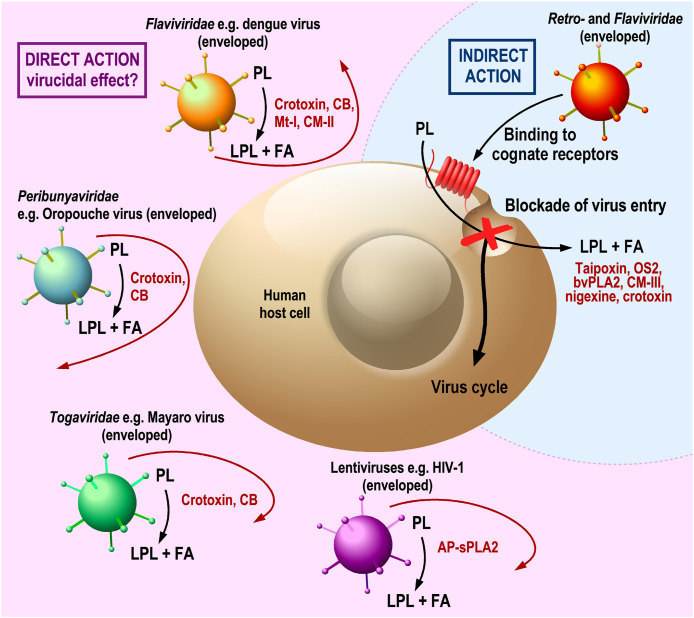
More than two decades ago, before studies with endogenous sPLA2s on viruses, it was shown that several venom sPLA2s exhibit a potent antiviral activity against HIV-1 in vitro [21] (Table 2 and Fig. 2 ). In this study, the bee venom sPLA2 (bvPLA2) and three snake venom sPLA2s were found to be potent inhibitors of HIV-1 replication (IC50 < 1 nM). hGIB and hGIIA sPLA2s did not show such inhibitory effects while other human sPLA2s could not be tested in those days, because they were either unknown or not available as recombinant pure proteins [4,5]. Four venom sPLA2s among 11 enzymes — taipoxin from the Australian coastal taipan (Oxyuranus scutellatus scutellatus), the basic sPLA2 (CM-III) from the Mozambique spitting cobra (Naja mossambica mossambica), nigexine from the black-necked spitting cobra (Naja nigricollis), and bvPLA2 from honey bee (Apis mellifera) — were able to protect human primary blood leukocytes from infection by various HIV-1 strains [21]. The observed inhibition resulted neither from a direct virucidal effect nor from a cytotoxic effect on host cells. Time-of-addition experiments monitoring the antiviral effect of sPLA2 after the virus was added to host cells showed that the inhibitory sPLA2s did not interfere with physical binding of the virus to the cell surface but blocked virus entry, possibly by interfering with the complex fusion step between viral envelope and plasma membrane. Accordingly, the inhibitory sPLA2s were effective when added to cells before the virion uncoating step and dissociation of the reverse transcriptase complex from host cell membranes, but were ineffective after these steps (Fig. 2). In a follow-up study, a variant of HIV-1 was selected from a mixture of HIV-1 particles present in primary HIV isolates for its resistance to bvPLA2 inhibition [103]. It turned out that this variant, HIVRBV-3, could escape from inhibition of cell entry to increasing doses of bvPLA2 by evolving or selecting a particular envelope glycoprotein mutated in the N-terminal region and the V1–V2 loop extensions [103]. These mutations allowed the HIV-1 variant to infect cells by a completely different clathrin endosomal-dependent pathway and to become resistant to inhibition by bvPLA2 by about 100-fold, when compared to the original virus. In an effort to identify the region of bvPLA2 involved in its inhibitory effect, a series of bvPLA2 peptides were screened, and an N-terminal 15-amino acid peptide was found to block entry of T-tropic HIV-1 strains into host cells, obviously without requiring enzyme catalytic activity. However, surprisingly, the mechanism of action of this peptide was different from that of the whole bvPLA2 molecule, as the peptide selectively inhibited T- but not M-tropic HIV-1 viruses by interfering with binding of HIV-1 to the CXCR4 chemokine coreceptor on target cells [93]. Conversely, bvPLA2 was active on both T- and M-tropic HIV-1 strains and did not bind to the CXCR4 co-receptor. Finally, additional structure-function studies using the neurotoxic snake venom sPLA2 OS2 (from Oxyuranus scutellatus scutellatus venom), which was also effective at inhibiting HIV-1, showed that the N-terminal region of OS2 and its enzymatic activity were both important to inhibit HIV-1 infection [92].
Table 2.
Antiviral activity of exogenous venom sPLA2s. Most of them are monomeric sPLA2s homologous to mammalian endogenous sPLA2s. They belong to groups IA, IB, IIA or III according to the historical classification of sPLA2s [1]. Heterodimeric crotoxin is composed of a basic GIIA sPLA2 (CB) and an acidic (CA) enzymatically-inactive subunit of three peptides derived from a GIIA sPLA2 precursor. Taipoxin is a heterotrimeric protein whose α and β subunits are GIA sPLA2s, and with the γ subunit similar to a GIB pro-sPLA2. D49 and K49 denotes enzymatically-active and -inactive sPLA2 variants, respectively, due to a substitution of the conserved aspartic acid residue at position 49 in the Calcium-binding loop (binding of the Ca2+ cofactor is needed for catalytic activity) of enzymatically-active sPLA2s with a lysine residue.
| Exogenous sPLA2 | Venom source | Type of viruses tested | Proposed mechanism of antiviral activity | Dependence on enzymatic activity | Reference |
|---|---|---|---|---|---|
| bvPLA2, CM-III, nigexine, taipoxin and OS2 | Apis mellifera, Naja mossambica mossambica, Naja nigricollis, and Oxyuranus scutellatus scutellatus (honey bee and three snake species) | Retroviridae (lentiviruses) | inhibition of HIV-1 entry step, inhibition of both M− and T-tropic strains | dependent | [21,93,103] |
| p3bv (15-aa bvPLA2 peptide) | Apis mellifera (honey bee) | Retroviridae | inhibition of the interaction between the CXCR4 chemokine co-receptor and HIV-1 T- but not M-tropic strains, thereby preventing virus binding to host cells | independent | [93] |
| AP-sPLA2 | Acanthaster planci (starfish) | Retroviridae | unknown, against HIV infection | not determined | [94] |
| Bl-D49 and K49 sPLA2s | Bothrops leucurus (snake) | Flaviviridae | preventing cells from being infected with dengue virus | not determined | [95] |
| crotoxin, crotoxin B subunit (CB PLA2), and recombinant CB1 and CB2 isoforms | Crotalus durissus terrificus (snake) | Flaviviridae, Peribunyaviridae, Togaviridae, Picornaviridae | possible virucidal activity against dengue, yellow fever and Zika viruses (all Flaviviridae) and other enveloped viruses from Peribunyaviridae and Togaviridae families by enzymatic cleavage of the virus lipid bilayer envelope that would cause a destabilization of the E proteins on the virus surface, leading to its inactivation | likely dependent | [[96], [97], [98], [99]] |
| crotoxin and its CB PLA2 subunit | Crotalus durissus terrificus (snake) | Flaviviridae | possible virucidal activity against hepatitis C virus (Flaviviridae) and/or interference with virus entry into host cells | not determined | [100] |
| CM-II | Naja mossambica mossambica (snake) | Flaviviridae, Retroviridae, Coronaviridae, Togaviridae, Herpesviridae, Orthomyxoviridae, Paramyxoviridae, Picornaviridae, Rhabdoviridae | possible specific virucidal activity against hepatitis C, dengue and Japanese encephalitis viruses (all Flaviviridae) and other viruses whose envelope is derived from the endoplasmic reticulum membrane by attacking their membranes; but also virucidal against HIV-1 whose membrane buds from the plasma membrane | likely dependent | [101] |
| Mt-I (D49) and Mt-II (K49) | Bothrops asper (snake) | Flaviviridae, Herpesviridae, Orthomyxoviridae, Picornaviridae, Rhabdoviridae | possible virucidal activity against dengue and yellow fever viruses (both Flaviviridae) | dependent | [102] |
Fig. 2.
The antiviral action of exogenous sPLA2s in in vitro cell systems. Direct action: certain venom sPLA2s may exert an antiviral activity by direct hydrolysis of the lipid viral envelope (virucidal effect?). The surface of various enveloped viruses, with the lipid membrane budding from different host cell membranes, is shown by different colours (orange, originating from the endoplasmic reticulum; blue-green, from the Golgi apparatus; green, from the plasma membrane; violet, from the plasma membrane (e.g. HIV-1) or endoplasmic reticulum). Indirect action: venom sPLA2s may exert antiviral activity by binding and hydrolysis of host cell membranes, thereby inhibiting one or several of the first steps of viral infection: from binding of viruses to entry or virus-driven lipid metabolism. The dashed line separates both direct and indirect sPLA2 actions on cells. A red curved arrow denotes the unsuccessful attack of a virus on the host cell as a result of the sPLA2 action. AP-sPLA2, a catalytically-active sPLA2 from Acanthaster planci starfish; CB, crotoxin B subunit; CM-II and CM-III, catalytically-active sPLA2s from Naja mossambica mossambica snake venom; Mt-I, a catalytically-active sPLA2 from Bothrops asper snake venom. Other abbreviations used are the same as those in Fig. 1 and Table 2.
During the last decade, several other studies have been carried out, showing a broad antiviral activity of different snake venom sPLA2s (Table 2 and Fig. 2). From these reports, it is difficult to unequivocally conclude that enzymatic activity of an sPLA2 is absolutely necessary for the antiviral effect observed. For example, both catalytically-active (D49) and catalytically-inactive (K49) sPLA2s, isolated from the venom of the Bahia lancehead (Bothrops leucurus), seemed to show similar antiviral activity in a cellular model of infection by the dengue virus [95]. However, since no dose-response curves to measure the relative IC50 values between D49 and K49 sPLA2s were performed, it is difficult to compare the relative activity of the two types of sPLA2s and exclude the possibility of a minor contamination of the K49 sPLA2 preparation with the D49 active sPLA2 purified from the same venom. Furthermore, some reports claimed that sPLA2s exert a direct virucidal activity but this was not conclusively demonstrated by either measuring the direct hydrolytic activity of the sPLA2 on viral particles or performing lipidomics analyses, or by washing out the sPLA2 after the incubation of viruses with this latter, or by addition of an sPLA2 inhibitor that would prevent the subsequent action of the sPLA2 on cells at the time of addition of the virus–sPLA2 mixture to host cells.
It was reported that crotoxin from the South American rattlesnake (Crotalus durissus terrificus) exhibits antiviral activity against dengue and yellow fever viruses [96]. In addition to preventing the cells from viral infections, the authors observed a pronounced effect of crotoxin when preincubated with the virus particles, suggesting a virucidal effect. However, the sPLA2–virus mixture was not separated before addition to cells, leaving open the possibility that the sPLA2 acts in a subsequent step of infection, for instance by interfering with the cell machinery during virus binding or entry. Crotoxin B (CB), the catalytically-active subunit of heterodimeric crotoxin, appeared to lose antiviral activity when its enzymatic activity is inhibited [97,98]. The virucidal action of crotoxin may be the result of hydrolysis of glycerophospholipids on the virus envelope, leading to a disruption of the lipid bilayer and destabilization of envelope (E) proteins on the viral surface. Molecular dynamics simulations suggested that crotoxin may gain access to the dengue virus lipid bilayer through the pores found on each of the twenty 3-fold vertices in the E protein shell on the virus surface [98]. However, definitive experimental evidence supporting this scenario is lacking. Crotoxin and its CB subunit had antiviral activity not only against Flaviviridae viruses (such as dengue, yellow fever and Zika) but also against Peribunyaviridae and Togaviridae viruses whose envelopes are different, originating from Golgi and plasma membranes, respectively. For example, two recombinant enzymatically active CB variants, CB1 and CB2, showed antiviral effects, not only against dengue, yellow fever and Zika viruses, but also against chikungunya virus (Togaviridae) [99]. In a separate study [100], crotoxin and its CB subunit were effective against in vitro infection with hepatitis C virus (Flaviviridae). The results indicated that CB blocks viral infection by action on the virus particle and host cells [100]. Thus, crotoxin and its CB subunit exert antiviral effects by possibly acting at different stages of the virus life cycle.
Mt-I, a myotoxic sPLA2 from terciopelo (Bothrops asper) snake venom was shown to exert antiviral activity against the enveloped viruses of Flaviviridae family, similar to that of crotoxin and CB [102]. Interestingly, the results showed that Mt-I, which is catalytically active (D49), was approximately 1,000-fold more potent than its catalytically inactive variant Mt-II (K49), suggesting a critical role of the sPLA2 enzymatic activity in the antiviral effect of Mt-I sPLA2.
An interesting finding resulted from the study of the antiviral action of the CM-II sPLA2 isolated from the venom of the Mozambique spitting cobra (Naja mossambica mossambica) against different types of viruses [101]. CM-II possesses potent antiviral activity (IC50 values of 0.03–1.3 ng/ml, i.e. less than 1 nM) against hepatitis C, dengue and Japanese encephalitis viruses, all from the Flaviviridae family, whose viral particles bud from the endoplasmic reticulum. In contrast, CM-II was virtually ineffective against viruses that bud either from the plasma membrane (Sindbis, influenza and Sendai viruses) or the trans-Golgi network (herpes simplex virus). One exception was HIV-1, which was inhibited by CM-II with an IC50 value of 5.4 ng/ml, a finding in line with the IC50 value measured for the analogous CM-III sPLA2 in an earlier study [21]. The potent antiviral activity of CM-II against hepatitis C and dengue viruses was inhibited by the broadly specific sPLA2 inhibitor manoalide, suggesting that enzymatic activity is necessary. Thus, based on differences in the physicochemical properties of the phospholipid bilayers of viruses and host cells, broad-spectrum antiviral sPLA2s may be developed to specifically target viral envelope lipid bilayers derived from the endoplasmic reticulum without targeting the lipid bilayers of host cell membranes.
Most recently, AP-sPLA2, isolated from the crown-of-thorns starfish (Acanthaster planci) was shown to exert in vitro activity against HIV infection of peripheral blood mononuclear cells [94]. The authors hypothesized but did not demonstrate that AP-sPLA2, like endogenous GX sPLA2 [24], directly hydrolyses the HIV phospholipid bilayer envelope which causes the virus to become inactive.
Overall, these studies have shown that several but not all venom exogenous sPLA2s can exert potent and broadly specific antiviral effects against different viruses by multiple mechanisms, from a possible direct virucidal action to more complex interplay between sPLA2, virus and host cells, from primary binding to the plasma membrane and specific receptors to physical virus entry, membrane fusion, internalization, multiplication and budding steps. In most cases, the exact mechanism of action, which may even be miscellaneous for a single sPLA2 acting on different viruses, remain to be discovered or at least ascertained by well-designed experiments. Furthermore, a major limitation is that all of these studies were performed only in vitro in cellular models of viral infection. Their therapeutic potential should be confirmed by performing in vivo experiments to provide a pathophysiological relevance of the findings.
5. Conclusion and future perspectives
sPLA2s constitute an important family of both enzymatically active and inactive proteins with a wide spectrum of activities whose biological functions, including antiviral action, have not been completely elucidated. Endogenous sPLA2s play an important but complex role in the innate immune system, sometimes defensive, sometimes offensive [8,9,104]. These yin-yang roles, for instance the proviral or antiviral effects of sPLA2s, are sometimes observed when a particular biological system is analysed in in vitro versus in vivo conditions, and as such is difficult to understand as a whole. Furthermore, it is difficult to definitely conclude from the current studies whether a certain sPLA2 is directly or indirectly influencing the virus life cycle, from direct virucidal effect to indirect effect on the binding, entry, or even budding steps of viruses to host cells (Fig. 1, Fig. 2). As a great deal of studies presented above has reported on the antiviral effects of sPLA2s by using in vitro cellular models of viral infections, there is a need to perform the corresponding in vivo experiments. Only in vivo results will expose the real antiviral potential of sPLA2s, acting either as therapeutic molecules or as “offensive” molecules whose activity would need to be hampered by active site-specific inhibitors or inhibitory antibodies. Most intensively, efforts should be concentrated on the most potent sPLA2 representatives acting on the virus life cycle, such as GIII, GV and GX sPLA2s, which may act on both host cell membranes and the viral envelope that has a lipid membrane derived from host cells, yet with subtle differences in their membrane lipid composition and biophysics. sPLA2s may also play an indirect role in the antiviral adaptive immune response where, interestingly, some sPLA2s like GX but also GIID sPLA2 have a negative (proviral) effect on the viral infection by H1N1 and SARS-coronavirus adapted to mouse strains [27,28]. In addition, it has been recently shown that GIB sPLA2 is a new player in lentiviral infections, where it may exert a proviral role by inducing CD4+ T cell anergy and thereby participate to the pathophysiology of HIV infection [56]. Here, the specific or combined inhibition of involved sPLA2s may be beneficial to prevent viral infection. Exogenous sPLA2s, mostly but not exclusively of snake venom origin, also display antiviral effect that may be mediated by their enzymatic activity to either directly attack the host-derived membranes of certain enveloped viruses or interfere with the viral cell cycle at different steps. Whether these sPLA2s may be used as antiviral agents in vivo remains to be demonstrated, considering of course their toxicities.
Future research on the antiviral potential of sPLA2s may thus proceed in four major directions. (1) Confirmation or disproval of the promising in vitro antiviral effects of certain venom and endogenous sPLA2s by conducting appropriate in vivo studies. (2) Description of the exact molecular mechanism(s) of direct or indirect antiviral activity of the various sPLA2s, as a starting point for development of new antiviral sPLA2-based therapies. This would also include unravelling of the indirect mechanisms by which GX and GIID sPLA2s (or possibly other isoforms) exacerbate H1N1 and coronavirus infections to pave the way for new therapeutic avenues. (3) Further description of the broadly specific antiviral role of endogenous and exogenous sPLA2s against various types of viruses, up to infection by SARS-CoV-2 virus, the etiological agent of the Covid-19 pandemic. (4) Exploration of the possibility that various sPLA2s such as GX or GIID may represent new biomarkers of severity in viral infections, as recently shown for the inflammatory-type GIIA sPLA2 in Covid-19 patients with severe disease [105].
Authors’ contributions
J.P. wrote the first drafts of the manuscript, figures and tables, F.B. and G.L. significantly upgraded and polished the main text, figures and tables, and I.K. checked the facts and reviewed the manuscript.
Acknowledgments
The authors sincerely thank Franck Aguila for expertise in graphic art and help in designing figures, and Dr. Roger H. Pain for critical reading of the manuscript draft. This work was supported by the Slovenian Research Agency grant P1-0207 to I.K., and by funding from the Centre National de la Recherche Scientifique (CNRS) to G.L.
References
- 1.Dennis E.A., Cao J., Hsu Y.H., Magrioti V., Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. Recent progress in phospholipase A₂ research: from cells to animals to humans. Prog. Lipid Res. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Lambeau G., Gelb M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 4.Valentin E., Lambeau G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochim. Biophys. Acta. 2000;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 5.Valentin E., Lambeau G. What can venom phospholipases A2 tell us about the functional diversity of mammalian secreted phospholipases A2? Biochimie. 2000;82:815–831. doi: 10.1016/s0300-9084(00)01168-8. [DOI] [PubMed] [Google Scholar]
- 6.Pungerčar J., Križaj I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2. Toxicon. 2007;50:871–892. doi: 10.1016/j.toxicon.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Lomonte B., Križaj I. In: Handbook of Venoms and Toxins of Reptiles. second ed. Mackessy Stephen P., editor. CRC Press/Taylor and Francis Group; 2021. Snake venom phospholipase A2 toxins; pp. 389–411. [Google Scholar]
- 8.Murakami M., Taketomi Y., Girard C., Yamamoto K., Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: lessons from transgenic and knockout mice. Biochimie. 2010;92:561–582. doi: 10.1016/j.biochi.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M., Sato H., Miki Y., Yamamoto K., Taketomi Y. A new era of secreted phospholipase A₂. J. Lipid Res. 2015;56:1248–1261. doi: 10.1194/jlr.R058123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez J.M., Lomonte B. Phospholipases A2, Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon. 2013;62:27–39. doi: 10.1016/j.toxicon.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Carpena M., Nuñez-Estevez B., Soria-Lopez A., Simal-Gandara J. Bee venom: an updating review of its bioactive molecules and its health applications. Nutrients. 2020;12:3360. doi: 10.3390/nu12113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Šribar J., Oberčkal J., Križaj I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2: an update. Toxicon. 2014;89:9–16. doi: 10.1016/j.toxicon.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Sobrinho J.C., Simões-Silva R., Holanda R.J., Alfonso J., Gomez A.F., Zanchi F.B., Moreira-Dill L.S., Grabner A.N., Zuliani J.P., Calderon L.A., Soares A.M. Antitumoral potential of snake venom phospholipases A2 and synthetic peptides. Curr. Pharmaceut. Biotechnol. 2016;17:1201–1212. doi: 10.2174/1389201017666160808154250. [DOI] [PubMed] [Google Scholar]
- 14.Lee G., Bae H. Bee venom phospholipase A2: yesterday's enemy becomes today's friend. Toxins. 2016;8:48. doi: 10.3390/toxins8020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zambelli V.O., Picolo G., Fernandes C.A.H., Fontes M.R.M., Cury Y. Secreted phospholipases A₂ from animal venoms in pain and analgesia. Toxins. 2017;9:406. doi: 10.3390/toxins9120406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida J.R., Palacios A.L.V., Patiño R.S.P., Mendes B., Teixeira C.A.S., Gomes P., da Silva S.L. Harnessing snake venom phospholipases A2 to novel approaches for overcoming antibiotic resistance. Drug Dev. Res. 2019;80:68–85. doi: 10.1002/ddr.21456. [DOI] [PubMed] [Google Scholar]
- 17.Nevalainen T.J., Graham G.G., Scott K.F. Antibacterial actions of secreted phospholipases A2. Review, Biochim. Biophys. Acta. 2008;1781:1–9. doi: 10.1016/j.bbalip.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Weiss J.P. Molecular determinants of bacterial sensitivity and resistance to mammalian group IIA phospholipase A2. Biochim. Biophys. Acta. 2015;1848:3072–3077. doi: 10.1016/j.bbamem.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guillaume C., Payre C., Jemel I., Jeammet L., Bezzine S., Naika G.S., Bollinger J., Grellier P., Gelb M.H., Schrevel J., Lambeau G., Deregnaucourt C. In vitro anti-Plasmodium falciparum properties of the full set of human secreted phospholipases A2. Infect. Immun. 2015;83:2453–2465. doi: 10.1128/IAI.02474-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teixeira S.C., Borges B.C., Oliveira V.Q., Carregosa L.S., Bastos L.A., Santos I.A., Jardim A.C.G., Melo F.F., Freitas L.M., Rodrigues V.M., Lopes D.S. Insights into the antiviral activity of phospholipases A2 (PLA2s) from snake venoms. Int. J. Biol. Macromol. 2020;164:616–625. doi: 10.1016/j.ijbiomac.2020.07.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenard D., Lambeau G., Valentin E., Lefebvre J.C., Lazdunski M., Doglio A. Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Invest. 1999;104:611–618. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsuishi M., Masuda S., Kudo I., Murakami M. Group V and X secretory phospholipase A2 prevents adenoviral infection in mammalian cells. Biochem. J. 2006;393:97–106. doi: 10.1042/BJ20050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsuishi M., Masuda S., Kudo I., Murakami M. Human group III phospholipase A2 suppresses adenovirus infection into host cells. Evidence that group III, V and X phospholipase A2s act on distinct cellular phospholipid molecular species. Biochim. Biophys. Acta. 2007;1771:1389–1396. doi: 10.1016/j.bbalip.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Kim J.O., Chakrabarti B.K., Guha-Niyogi A., Louder M.K., Mascola J.R., Ganesh L., Nabel G.J. Lysis of human immunodeficiency virus type 1 by a specific secreted human phospholipase A2. J. Virol. 2007;81:1444–1450. doi: 10.1128/JVI.01790-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nevalainen T.J., Losacker W. Serum phospholipase A2 in dengue. J. Infect. 1997;35:251–252. doi: 10.1016/s0163-4453(97)92966-2. [DOI] [PubMed] [Google Scholar]
- 26.Ito M., Ishikawa Y., Kiguchi H., Komiyama K., Murakami M., Kudo I., Akasaka Y., Ishii T. Distribution of type V secretory phospholipase A2 expression in human hepatocytes damaged by liver disease. J. Gastroenterol. Hepatol. 2004;19:1140–1149. doi: 10.1111/j.1440-1746.2004.03435.x. [DOI] [PubMed] [Google Scholar]
- 27.Kelvin A.A., Degousee N., Banner D., Stefanski E., Leomicronn A.J., Angoulvant D., Paquette S.G., Huang S.S., Danesh A., Robbins C.S., Noyan H., Husain M., Lambeau G., Gelb M., Kelvin D.J., Rubin B.B. Lack of group X secreted phospholipase A2 increases survival following pandemic H1N1 influenza infection. Virology. 2014;454–455:78–92. doi: 10.1016/j.virol.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijay R., Hua X., Meyerholz D.K., Miki Y., Yamamoto K., Gelb M., Murakami M., Perlman S. Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome-CoV infection. J. Exp. Med. 2015;212:1851–1868. doi: 10.1084/jem.20150632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouault M., Bollinger J.G., Lazdunski M., Gelb M.H., Lambeau G. Novel mammalian group XII secreted phospholipase A2 lacking enzymatic activity. Biochemistry. 2003;42:11494–11503. doi: 10.1021/bi0349930. [DOI] [PubMed] [Google Scholar]
- 30.Singer A.G., Ghomashchi F., Le Calvez C., Bollinger J., Bezzine S., Rouault M., Sadilek M., Nguyen E., Lazdunski M., Lambeau G., Gelb M.H. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto K., Miki Y., Sato H., Murase R., Taketomi Y., Murakami M. Secreted phospholipase A2 specificity on natural membrane phospholipids. Methods Enzymol. 2017;583:101–117. doi: 10.1016/bs.mie.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q., Yang M., Fu X., Liu R., Sun C., Pan H., Wong C.W., Guan M. Activation of farnesoid X receptor promotes triglycerides lowering by suppressing phospholipase A2 G12B expression. Mol. Cell. Endocrinol. 2016;436:93–101. doi: 10.1016/j.mce.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X., Jones S.M., Yamoah E.N., Lundberg Y.W. Otoconin-90 deletion leads to imbalance but normal hearing: a comparison with other otoconia mutants. Neuroscience. 2008;153:289–299. doi: 10.1016/j.neuroscience.2008.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Kowalski P.E., Thalmann I., Ornitz D.M., Mager D.L., Thalmann R. Otoconin-90, the mammalian otoconial matrix protein, contains two domains of homology to secretory phospholipase A2. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15345–15350. doi: 10.1073/pnas.95.26.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verpy E., Leibovici M., Petit C. Characterization of otoconin-95, the major protein of murine otoconia, provides insights into the formation of these inner ear biominerals. Proc. Natl. Acad. Sci. U.S.A. 1999;96:529–534. doi: 10.1073/pnas.96.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami M., Taketomi Y., Miki Y., Sato H., Yamamoto K., Lambeau G. Vol. 107. 2014. pp. 105–113. (Emerging roles of secreted phospholipase A2 enzymes, the 3rd edition, Biochimie). [DOI] [PubMed] [Google Scholar]
- 37.Murakami M., Sato H., Taketomi Y. Updating phospholipase A2 biology. Biomolecules. 2020;10:1457. doi: 10.3390/biom10101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tischfield J.A., Xia Y.R., Shih D.M., Klisak I., Chen J., Engle S.J., Siakotos A.N., Winstead M.V., Seilhamer J.J., Allamand V., Gyapay G., Lusis A.J. Low-molecular-weight, calcium-dependent phospholipase A2 genes are linked and map to homologous chromosome regions in mouse and human. Genomics. 1996;32:328–333. doi: 10.1006/geno.1996.0126. [DOI] [PubMed] [Google Scholar]
- 39.Chen J., Shao C., Lazar V., Srivastava C.H., Lee W.H., Tischfield J.A. Localization of group IIc low molecular weight phospholipase A2 mRNA to meiotic cells in the mouse. J. Cell. Biochem. 1997;64:369–375. doi: 10.1002/(sici)1097-4644(19970301)64:3<369::aid-jcb3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 40.Murakami M., Miki Y., Sato H., Murase R., Taketomi Y., Yamamoto K. Group IID, IIE, IIF and III secreted phospholipase A2s. Biochim. Biophys. Acta – Mol. Cell Biol. Lipids. 2019;1864:803–818. doi: 10.1016/j.bbalip.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentin E., Ghomashchi F., Gelb M.H., Lazdunski M., Lambeau G. On the diversity of secreted phospholipases A2. Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes. J. Biol. Chem. 1999;274:31195–31202. doi: 10.1074/jbc.274.44.31195. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki N., Ishizaki J., Yokota Y., Higashino K., Ono T., Ikeda M., Fujii N., Kawamoto K., Hanasaki K. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipases A2. J. Biol. Chem. 2000;275:5785–5793. doi: 10.1074/jbc.275.8.5785. [DOI] [PubMed] [Google Scholar]
- 43.Escoffier J., Jemel I., Tanemoto A., Taketomi Y., Payré C., Coatrieux C., Sato H., Yamamoto K., Masuda S., Pernet-Gallay K., Pierre V., Hara S., Murakami M., De Waard M., Lambeau G., Arnoult C. Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J. Clin. Invest. 2010;120:1415–1428. doi: 10.1172/JCI40494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li K., Jin J.Y., Chen W.Y., Shi Q.X., Ni Y., Roldan E.R. Secretory phospholipase A2 group IID is involved in progesterone-induced acrosomal exocytosis of human spermatozoa. J. Androl. 2012;33:975–983. doi: 10.2164/jandrol.111.014886. [DOI] [PubMed] [Google Scholar]
- 45.Anfuso C.D., Olivieri M., Bellanca S., Salmeri M., Motta C., Scalia M., Satriano C., La Vignera S., Burrello N., Caporarello N., Lupo G., Calogero A.E. Asthenozoospermia and membrane remodeling enzymes: a new role for phospholipase A2. Andrology. 2015;3:1173–1182. doi: 10.1111/andr.12101. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy B.P., Payette P., Mudgett J., Vadas P., Pruzanski W., Kwan M., Tang C., Rancourt D.E., Cromlish W.A. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J. Biol. Chem. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- 47.Dore E., Boilard E. Roles of secreted phospholipase A2 group IIA in inflammation and host defense. Biochim. Biophys. Acta – Mol. Cell Biol. Lipids. 2019;1864:789–802. doi: 10.1016/j.bbalip.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Brglez V., Lambeau G., Petan T. Secreted phospholipases A2 in cancer: diverse mechanisms of action. Biochimie. 2014;107:114–123. doi: 10.1016/j.biochi.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Schewe M., Franken P.F., Sacchetti A., Schmitt M., Joosten R., Böttcher R., van Royen M.E., Jeammet L., Payré C., Scott P.M., Webb N.R., Gelb M., Cormier R.T., Lambeau G., Fodde R. Secreted phospholipases A2 are intestinal stem cell niche factors with distinct roles in homeostasis, inflammation and cancer. Cell Stem Cell. 2016;19:38–51. doi: 10.1016/j.stem.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 50.Masuda S., Murakami M., Mitsuishi M., Komiyama K., Ishikawa Y., Ishii T., Kudo I. Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells. Biochem. J. 2005;387:27–38. doi: 10.1042/BJ20041307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masuda S., Murakami M., Ishikawa Y., Ishii T., Kudo I. Diverse cellular localizations of secretory phospholipase A2 enzymes in several human tissues. Biochim. Biophys. Acta. 2005;1736:200–210. doi: 10.1016/j.bbalip.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Limou S., Coulonges C., Foglio M., Heath S., Diop G., Leclerc S., Hirtzig T., Spadoni J.L., Therwath A., Lambeau G., Gut I., Zagury J.F. Exploration of associations between phospholipase A2 gene family polymorphisms and AIDS progression using the SNPlex method. Biomed. Pharmacother. 2008;62:31–40. doi: 10.1016/j.biopha.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Holmes M.V., Simon T., Exeter H.J., Folkersen L., Asselbergs F.W., Guardiola M., Cooper J.A., Palmen J., Hubacek J.A., Carruthers K.F., Horne B.D., Brunisholz K.D., Mega J.L., van Iperen E.P., Li M., Leusink M., Trompet S., Verschuren J.J., Hovingh G.K., Dehghan A., Nelson C.P., Kotti S., Danchin N., Scholz M., Haase C.L., Rothenbacher D., Swerdlow D.I., Kuchenbaecker K.B., Staines-Urias E., Goel A., van 't Hooft F., Gertow K., de Faire U., Panayiotou A.G., Tremoli E., Baldassarre D., Veglia F., Holdt L.M., Beutner F., Gansevoort R.T., Navis G.J., Mateo Leach I., Breitling L.P., Brenner H., Thiery J., Dallmeier D., Franco-Cereceda A., Boer J.M., Stephens J.W., Hofker M.H., Tedgui A., Hofman A., Uitterlinden A.G., Adamkova V., Pitha J., Onland-Moret N.C., Cramer M.J., Nathoe H.M., Spiering W., Klungel O.H., Kumari M., Whincup P.H., Morrow D.A., Braund P.S., Hall A.S., Olsson A.G., Doevendans P.A., Trip M.D., Tobin M.D., Hamsten A., Watkins H., Koenig W., Nicolaides A.N., Teupser D., Day I.N., Carlquist J.F., Gaunt T.R., Ford I., Sattar N., Tsimikas S., Schwartz G.G., Lawlor D.A., Morris R.W., Sandhu M.S., Poledne R., Maitland-van der Zee A.H., Khaw K.T., Keating B.J., van der Harst P., Price J.F., Mehta S.R., Yusuf S., Witteman J.C., Franco O.H., Jukema J.W., de Knijff P., Tybjaerg-Hansen A., Rader D.J., Farrall M., Samani N.J., Kivimaki M., Fox K.A., Humphries S.E., Anderson J.L., Boekholdt S.M., Palmer T.M., Eriksson P., Pare G., Hingorani A.D., Sabatine M.S., Mallat Z., Casas J.P., Talmud P.J. Secretory phospholipase A2-IIA and cardiovascular disease: a Mendelian randomization study. J. Am. Coll. Cardiol. 2013;62:1966–1976. doi: 10.1016/j.jacc.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guardiola M., Exeter H.J., Perret C., Folkersen L., Van't Hooft F., Eriksson P., Franco-Cereceda A., Paulsson-Berne G., Palmen J., Li K., Cooper J.A., Khaw K.T., Mallat Z., Ninio E., Karabina S.A., Humphries S.E., Boekholdt S.M., Holmes M.V., Talmud P.J. PLA2G10 Gene variants, sPLA2 activity, and coronary heart disease risk. Circ. Cardiovasc. Genet. 2015;8:356–362. doi: 10.1161/CIRCGENETICS.114.000633. [DOI] [PubMed] [Google Scholar]
- 55.Akinkuolie A.O., Lawler P.R., Chu A.Y., Caulfield M., Mu J., Ding B., Nyberg F., Glynn R.J., Ridker P.M., Hurt-Camejo E., Chasman D.I., Mora S. Group IIA secretory phospholipase A2, vascular inflammation, and incident cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2019;39:1182–1190. doi: 10.1161/ATVBAHA.118.311894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pothlichet J., Rose T., Bugault F., Jeammet L., Meola A., Haouz A., Saul F., Geny D., Alcami J., Ruiz-Mateos E., Teyton L., Lambeau G., Theze J. PLA2G1B is involved in CD4 anergy and CD4 lymphopenia in HIV-infected patients. J. Clin. Invest. 2020;130:2872–2887. doi: 10.1172/JCI131842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fourcade O., Simon M.F., Viode C., Rugani N., Leballe F., Ragab A., Fournie B., Sarda L., Chap H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–927. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- 58.Murakami M., Koduri R.S., Enomoto A., Shimbara S., Seki M., Yoshihara K., Singer A., Valentin E., Ghomashchi F., Lambeau G., Gelb M.H., Kudo I. Distinct arachidonate-releasing functions of mammalian secreted phospholipases A2 in fibroblastic and mastocytoma cells through heparan sulfate shuttling and external plasma membrane membrane mechanisms. J. Biol. Chem. 2001;276:10083–10096. doi: 10.1074/jbc.M007877200. [DOI] [PubMed] [Google Scholar]
- 59.Mounier C.M., Ghomashchi F., Lindsay M.R., James S., Singer A.G., Parton R.G., Gelb M.H. Arachidonic acid release from mammalian cells transfected with human groups IIA and X secreted phospholipase A2 occurs predominantly during the secretory process and with the involvement of cytosolic phospholipase A2-alpha. J. Biol. Chem. 2004;279:25024–25038. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- 60.Pruzanski W., Lambeau G., Lazdunski M., Cho W., Kopilov J., Kuksis A. Differential hydrolysis of molecular species of lipoprotein phosphatidylcholine by groups IIA, V and X secretory phospholipases A2. Biochim. Biophys. Acta. 2005;1736:38–50. doi: 10.1016/j.bbalip.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Boudreau L.H., Duchez A.C., Cloutier N., Soulet D., Martin N., Bollinger J., Paré A., Rousseau M., Naika G.S., Lévesque T., Laflamme C., Marcoux G., Lambeau G., Farndale R.W., Pouliot M., Hamzeh-Cognasse H., Cognasse F., Garraud O., Nigrovic P.A., Guderley H., Lacroix S., Thibault L., Semple J.W., Gelb M.H., Boilard E. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jemel I., Ii H., Oslund R.C., Payré C., Dabert-Gay A.S., Douguet D., Chargui K., Scarzello S., Gelb M.H., Lambeau G. Group X secreted phospholipase A2 proenzyme is matured by a furin-like proprotein convertase and releases arachidonic acid inside of human HEK293 cells. J. Biol. Chem. 2011;286:36509–36521. doi: 10.1074/jbc.M111.268540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato H., Taketomi Y., Isogai Y., Miki Y., Yamamoto K., Masuda S., Hosono T., Arata S., Ishikawa Y., Ishii T., Kobayashi T., Nakanishi H., Ikeda K., Taguchi R., Hara S., Kudo I., Murakami M. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Invest. 2010;120:1400–1414. doi: 10.1172/JCI40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Entwistle L.J., Pelly V.S., Coomes S.M., Kannan Y., Perez-Lloret J., Czieso S., Silva Dos Santos M., MacRae J.I., Collinson L., Sesay A., Nikolov N., Metidji A., Helmby H., Hui D.Y., Wilson M.S. Epithelial-cell-derived phospholipase A2 group 1B is an endogenous anthelmintic. Cell Host Microbe. 2017;22:484–493. doi: 10.1016/j.chom.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muñoz-Sanjuan I., Brivanlou A.H. Induction of ectopic olfactory structures and bone morphogenetic protein inhibition by Rossy, a group XII secreted phospholipase A2. Mol. Cell Biol. 2005;25:3608–3619. doi: 10.1128/MCB.25.9.3608-3619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huhtinen H.T., Grönroos J.O., Grönroos J.M., Uksila J., Gelb M.H., Nevalainen T.J., Laine V.J. Antibacterial effects of human group IIA and group XIIA phospholipase A2 against Helicobacter pylori in vitro. APMIS. 2006;114:127–130. doi: 10.1111/j.1600-0463.2006.apm_330.x. [DOI] [PubMed] [Google Scholar]
- 67.Ee S.M., Lo Y.L., Shui G., Wenk M.R., Shin E.J., Kim H.C., Ong W.Y. Distribution of secretory phospholipase A2 XIIA in the brain and its role in lipid metabolism and cognition. Mol. Neurobiol. 2014;50:60–75. doi: 10.1007/s12035-014-8635-7. [DOI] [PubMed] [Google Scholar]
- 68.Peuravuori H., Kollanus S., Nevalainen T.J. Expression of group XIIA phospholipase A2 in human digestive organs. APMIS. 2014;122:1171–1177. doi: 10.1111/apm.12280. [DOI] [PubMed] [Google Scholar]
- 69.Wu Y., Raymond B., Goossens P.L., Njamkepo E., Guiso N., Paya M., Touqui L. Type-IIA secreted phospholipase A2 is an endogenous antibiotic-like protein of the host. Biochimie. 2010;92:583–587. doi: 10.1016/j.biochi.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 70.Koduri R.S., Grönroos J.O., Laine V.J., Le Calvez C., Lambeau G., Nevalainen T.J., Gelb M.H. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2002;277:5849–5857. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- 71.Dacheux M., Sinou V., Payre C., Jeammet L., Parzy D., Grellier P., Deregnaucourt C., Lambeau G. Antimalarial activity of human group IIA secreted phospholipase A2 in relation to enzymatic hydrolysis of oxidized lipoproteins. Infect. Immun. 2019;87 doi: 10.1128/IAI.00556-19. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balestrieri B., Maekawa A., Xing W., Gelb M.H., Katz H.R., Arm J.P. Group V secretory phospholipase A2 modulates phagosome maturation and regulates the innate immune response against Candida albicans. J. Immunol. 2009;182:4891–4898. doi: 10.4049/jimmunol.0803776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinrauch Y., Elsbach P., Madsen L.M., Foreman A., Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J. Clin. Invest. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elsbach P., Weiss J., Franson R.C., Beckerdite-Quagliata S., Schneider A., Harris L. Separation and purification of a potent bactericidal/permeability-increasing protein and a closely associated phospholipase A2 from rabbit polymorphonuclear leukocytes. Observations on their relationship. J. Biol. Chem. 1979;254:11000–11009. [PubMed] [Google Scholar]
- 75.Pernet E., Guillemot L., Burgel P.R., Martin C., Lambeau G., Sermet-Gaudelus I., Sands D., Leduc D., Morand P.C., Jeammet L., Chignard M., Wu Y., Touqui L. Pseudomonas aeruginosa eradicates Staphylococcus aureus by manipulating the host immunity. Nat. Commun. 2014;5:5105. doi: 10.1038/ncomms6105. [DOI] [PubMed] [Google Scholar]
- 76.Aloia R.C., Tian H., Jensen F.C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bezzine S., Koduri R.S., Valentin E., Murakami M., Kudo I., Ghomashchi F., Sadilek M., Lambeau G., Gelb M.H. Exogenously added human group X secreted phospholipase A2 but not the group IB, IIA, and V enzymes efficiently release arachidonic acid from adherent mammalian cells. J. Biol. Chem. 2000;275:3179–3191. doi: 10.1074/jbc.275.5.3179. [DOI] [PubMed] [Google Scholar]
- 78.Hanasaki K., Ono T., Saiga A., Morioka Y., Ikeda M., Kawamoto K., Higashino K., Nakano K., Yamada K., Ishizaki J., Arita H. Purified group X secretory phospholipase A2 induced prominent release of arachidonic acid from human myeloid leukemia cells. J. Biol. Chem. 1999;274:34203–34211. doi: 10.1074/jbc.274.48.34203. [DOI] [PubMed] [Google Scholar]
- 79.Brügger B., Krautkrämer E., Tibroni N., Munte C.E., Rauch S., Leibrecht I., Glass B., Breuer S., Geyer M., Kräusslich H.G., Kalbitzer H.R., Wieland F.T., Fackler O.T. Human immunodeficiency virus type 1 Nef protein modulates the lipid composition of virions and host cell membrane microdomains. Retrovirology. 2007;4:70. doi: 10.1186/1742-4690-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brügger B., Glass B., Haberkant P., Leibrecht I., Wieland F.T., Kräusslich H.G. The HIV lipidome: a raft with an unusual composition. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lorizate M., Sachsenheimer T., Glass B., Habermann A., Gerl M.J., Kräusslich H.G., Brügger B. Comparative lipidomics analysis of HIV-1 particles and their producer cell membrane in different cell lines. Cell Microbiol. 2013;15:292–304. doi: 10.1111/cmi.12101. [DOI] [PubMed] [Google Scholar]
- 82.Mounier C.M., Wendum D., Greenspan E., Flejou J.F., Rosenberg D.W., Lambeau G. Distinct expression pattern of the full set of secreted phospholipases A2 in human colorectal adenocarcinomas: sPLA2-III as a biomarker candidate. Br. J. Canc. 2008;98:587–595. doi: 10.1038/sj.bjc.6604184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Surrel F., Jemel I., Boilard E., Bollinger J.G., Payré C., Mounier C.M., Talvinen K.A., Laine V.J., Nevalainen T.J., Gelb M.H., Lambeau G. Group X phospholipase A2 stimulates the proliferation of colon cancer cells by producing various lipid mediators. Mol. Pharmacol. 2009;76:778–790. doi: 10.1124/mol.108.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valentin E., Koduri R.S., Scimeca J.-C., Carle G., Gelb M.H., Lazdunski M., Lambeau G. Cloning and recombinant expression of a novel mouse secreted phospholipase A2. J. Biol. Chem. 1999;274:19152–19160. doi: 10.1074/jbc.274.27.19152. [DOI] [PubMed] [Google Scholar]
- 85.Miki Y., Yamamoto K., Taketomi Y., Sato H., Shimo K., Kobayashi T., Ishikawa Y., Ishii T., Nakanishi H., Ikeda K., Taguchi R., Kabashima K., Arita M., Arai H., Lambeau G., Bollinger J.M., Hara S., Gelb M.H., Murakami M. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 2013;210:1217–1234. doi: 10.1084/jem.20121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serhan C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB. J. 2017;31:1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miki Y., Kidoguchi Y., Sato M., Taketomi Y., Taya C., Muramatsu K., Gelb M.H., Yamamoto K., Murakami M. Dual roles of group IID phospholipase A2 in inflammation and cancer. J. Biol. Chem. 2016;291:15588–15601. doi: 10.1074/jbc.M116.734624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shakhov A.N., Rubtsov A.V., Lyakhov I.G., Tumanov A.V., Nedospasov S.A. SPLASH (PLA2IID), a novel member of phospholipase A2 family, is associated with lymphotoxin deficiency. Genes. Immunol. 2000;1:191–199. doi: 10.1038/sj.gene.6363659. [DOI] [PubMed] [Google Scholar]
- 89.von Allmen C.E., Schmitz N., Bauer M., Hinton H.J., Kurrer M.O., Buser R.B., Gwerder M., Muntwiler S., Sparwasser T., Beerli R.R., Bachmann M.F. Secretory phospholipase A2-IID is an effector molecule of CD4+ CD25+ regulatory T cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11673–11678. doi: 10.1073/pnas.0812569106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murakami M., Yamamoto K., Miki Y., Murase R., Sato H., Taketomi Y. The roles of the secreted phospholipase A2 gene family in immunology. Adv. Immunol. 2016;132:91–134. doi: 10.1016/bs.ai.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng J., Meyerholz D., Wong L.R., Gelb M., Murakami M., Perlman S. Coronavirus-specific antibody production in middle-aged mice requires phospholipase A2G2D. J. Clin. Invest. 2021;131:147201. doi: 10.1172/JCI147201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rouault M., Rash L.D., Escoubas P., Boilard E., Bollinger J., Lomonte B., Maurin T., Guillaume C., Canaan S., Deregnaucourt C., Schrevel J., Doglio A., Gutierrez J.M., Lazdunski M., Gelb M.H., Lambeau G. Neurotoxicity and other pharmacological activities of the snake venom phospholipase A2 OS2: the N-terminal region is more important than enzymatic activity. Biochemistry. 2006;45:5800–5816. doi: 10.1021/bi060217r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fenard D., Lambeau G., Maurin T., Lefebvre J.C., Doglio A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001;60:341–347. doi: 10.1124/mol.60.2.341. [DOI] [PubMed] [Google Scholar]
- 94.Wijanarko A., Lischer K., Hermansyah H., Pratami D.K., Sahlan M. Antiviral activity of Acanthaster planci phospholipase A2 against human immunodeficiency virus. Vet. World. 2018;11:824–829. doi: 10.14202/vetworld.2018.824-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cecilio A.B., Caldas S., Oliveira R.A., Santos A.S., Richardson M., Naumann G.B., Schneider F.S., Alvarenga V.G., Estevão-Costa M.I., Fuly A.L., Eble J.A., Sanchez E.F. Molecular characterization of Lys49 and Asp49 phospholipases A₂ from snake venom and their antiviral activities against Dengue virus. Toxins. 2013;5:1780–1798. doi: 10.3390/toxins5101780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muller V.D., Russo R.R., Cintra A.C., Sartim M.A., Alves-Paiva R.M., Figueiredo L.T., Sampaio S.V., Aquino V.H. Crotoxin and phospholipases A₂ from Crotalus durissus terrificus showed antiviral activity against dengue and yellow fever viruses. Toxicon. 2012;59:507–515. doi: 10.1016/j.toxicon.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 97.Russo R.R., Muller V.D.M., Cintra A.C.O., Figueiredo L.T.M., Sampaio S.V., Aquino V.H. Phospholipase A2 crotoxin B isolated from the venom of Crotalus durissus terrificus exert antiviral effect against dengue virus and yellow fever virus through its catalytic activity. J. Virol. Antivir. Res. 2014;3:1. [Google Scholar]
- 98.Muller V.D., Soares R.O., dos Santos N.N., Jr., Trabuco A.C., Cintra A.C., Figueiredo L.T., Caliri A., Sampaio S.V., Aquino V.H. Phospholipase A2 isolated from the venom of Crotalus durissus terrificus inactivates dengue virus and other enveloped viruses by disrupting the viral envelope. PloS One. 2014;9 doi: 10.1371/journal.pone.0112351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Russo R.R., dos Santos N.N., Jr., Cintra A.C.O., Figueiredo L.T.M., Sampaio S.V., Aquino V.H. Expression, purification and virucidal activity of two recombinant isoforms of phospholipase A2 from Crotalus durissus terrificus venom. Arch. Virol. 2019;164:1159–1171. doi: 10.1007/s00705-019-04172-6. [DOI] [PubMed] [Google Scholar]
- 100.Shimizu J.F., Pereira C.M., Bittar C., Batista M.N., Campos G.R.F., da Silva S., Cintra A.C.O., Zothner C., Harris M., Sampaio S.V., Aquino V.H., Rahal P., Jardim A.C.G. Multiple effects of toxins isolated from Crotalus durissus terrificus on the hepatitis C virus life cycle. PloS One. 2017;12 doi: 10.1371/journal.pone.0187857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen M., Aoki-Utsubo C., Kameoka M., Deng L., Terada Y., Kamitani W., Sato K., Koyanagi Y., Hijikata M., Shindo K., Noda T., Kohara M., Hotta H. Broad-spectrum antiviral agents: secreted phospholipase A2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane. Sci. Rep. 2017;7:15931. doi: 10.1038/s41598-017-16130-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brenes H., Loria G.D., Lomonte B. Potent virucidal activity against Flaviviridae of a group IIA phospholipase A2 isolated from the venom of Bothrops asper. Biologicals. 2020;63:48–52. doi: 10.1016/j.biologicals.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Maurin T., Fenard D., Lambeau G., Doglio A. An envelope-determined endocytic route of viral entry allows HIV-1 to escape from secreted phospholipase A2 entry blockade. J. Mol. Biol. 2007;367:702–714. doi: 10.1016/j.jmb.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 104.Menschikowski M., Hagelgans A., Siegert G. Secretory phospholipase A2 of group IIA: is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases? Prostag. Other Lipid Mediat. 2006;79:1–33. doi: 10.1016/j.prostaglandins.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 105.Snider J.M., You J.K., Wang X., Snide A.J., Hallmark B., Seeds M.C., Sergeant S., Johnstone L., Wang Q., Sprissler R., Zhang H.H., Luberto C., Kew R.R., Hannun Y.A., McCall C.E., Yao G., Del Poeta M., Chilton F.H. Group IIA secreted phospholipase A2 plays a central role in the pathobiology of COVID-19. medRxiv [Preprint] 2021 doi: 10.1101/2021.02.22.21252237. Feb 23:2021.02.22.21252237. PMID: 33655264; PMCID: PMC7924289. [DOI] [PMC free article] [PubMed] [Google Scholar]