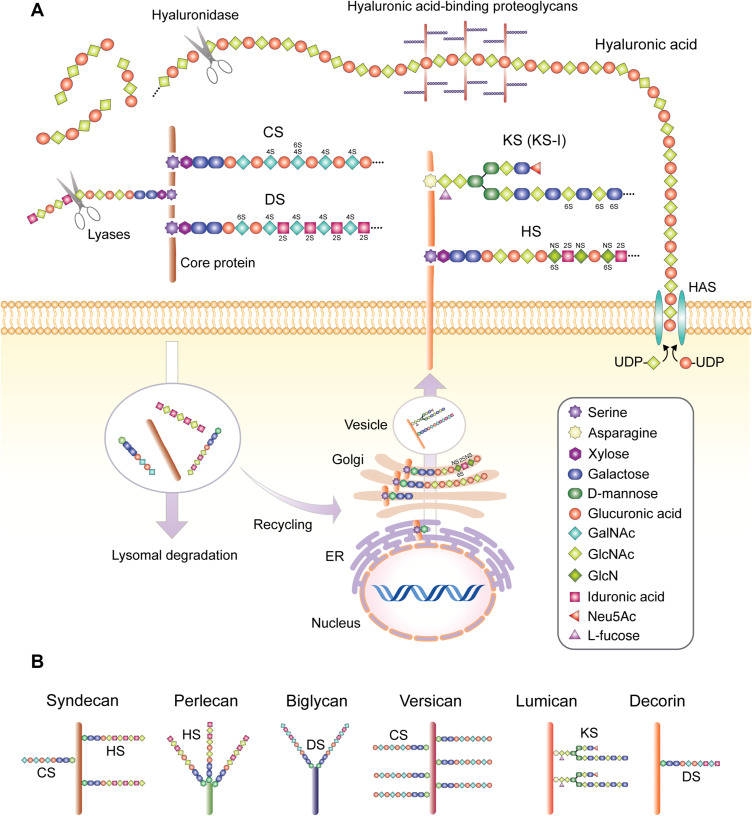
Figure 1.
(A) Structure of GAGs found in skin and synthesis processes. Key skin sulfated GAGs heparan sulfate (HS), dermatan sulfate (DS), chondroitin sulfate (CS) and keratin sulfate (KS, KS-I form depicted) are synthesized on a protein backbone with the xylose group attached in the endoplasmic reticulum (ER), followed by the sugar groups added in the Golgi apparatus, sulfation at the trans-Golgi and finally transportation to the plasma membrane via vesicles to form transmembrane units or excreted into the extracellular space, or more rarely located intracellularly (not depicted). The sulfated GAGs covalently bound to the core protein backbone create a proteoglycan unit, with differences in core proteins, the type of GAG bound to the protein, variations in the GAG sulfation sites and number of attached GAGs resulting in different proteoglycan families and considerable within-family diversity. These GAG chains can be degraded by lyases such as heparinase or chondroitinase, while the proteoglycan unit is subject to lysosomal degradation and intracellular recycling. Hyaluronic acid is synthesized by membrane-associated HAS enzymes, resulting in long HA chains which do not bind to a protein core, but can bind other proteoglycan complexes, which can be degraded by hyaluronidase. 2S, 4S, and 6S represent sulfation at 2-O, 4-O & 6-O positions, respectively. NS represents N-sulfated (B) proteoglycans that feature prominently in skin and their commonly associated GAG formations. Sizes not to scale.