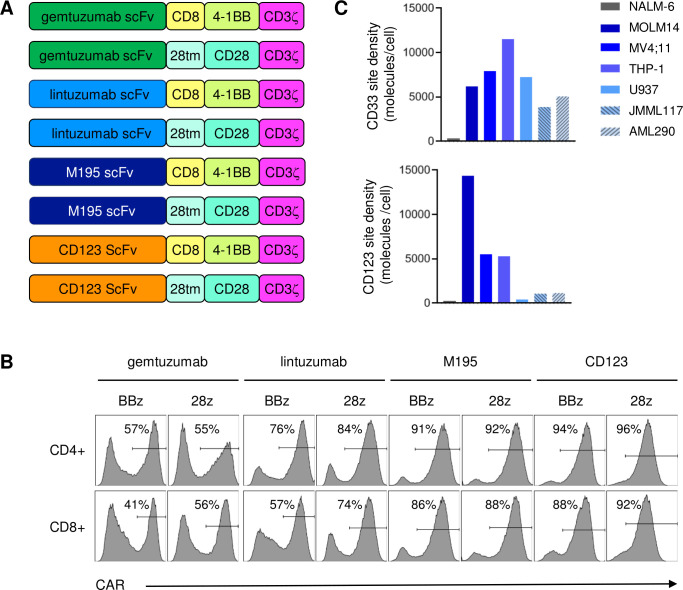
Figure 1.
CD33 chimeric antigen receptor construct (CD33CART) design and validation. (A) Schema of second-generation CD33-redirectedCAR constructs. Single-chain variable fragments (ScFvs) were derived from commercially available CD33 antibodies gemtuzumab ozogamicin (hP67.6; green), lintuzumab (SGN-33; light blue), and M195 (dark blue) and paired with a CD8 hinge or CD28 transmembrane (28tm) domain linker, 4-1BB or CD28 costimulatory endodomain, and a CD3ζ endodomain (designated BBz and 28z, respectively). CD123 CAR T cells with a 32 716 ScFv were used as a positive control for some studies. (B) Flow cytometry analysis of protein L expression demonstrating transduction efficiency of T cells transduced with gemtuzumab-based (gem), lintuzumab-based (lin), and M195-based CD33 CAR constructs or CD123 CAR constructs. (C) Flow cytometry quantification of CD33 site density (molecules/cell) on AML cell lines (MOLM14, MV4;11, THP-1, U937) and childhood acute myeloid leukemia (AML) patient-derived xenograft (PDX) models (JMML117, AML290) used for in vitro and/or in vivo testing of CD33CARTs. CD123 site density is shown as a comparator. The B-ALL cell line NALM-6 was used as a CD33-negative control.