Figure 7.
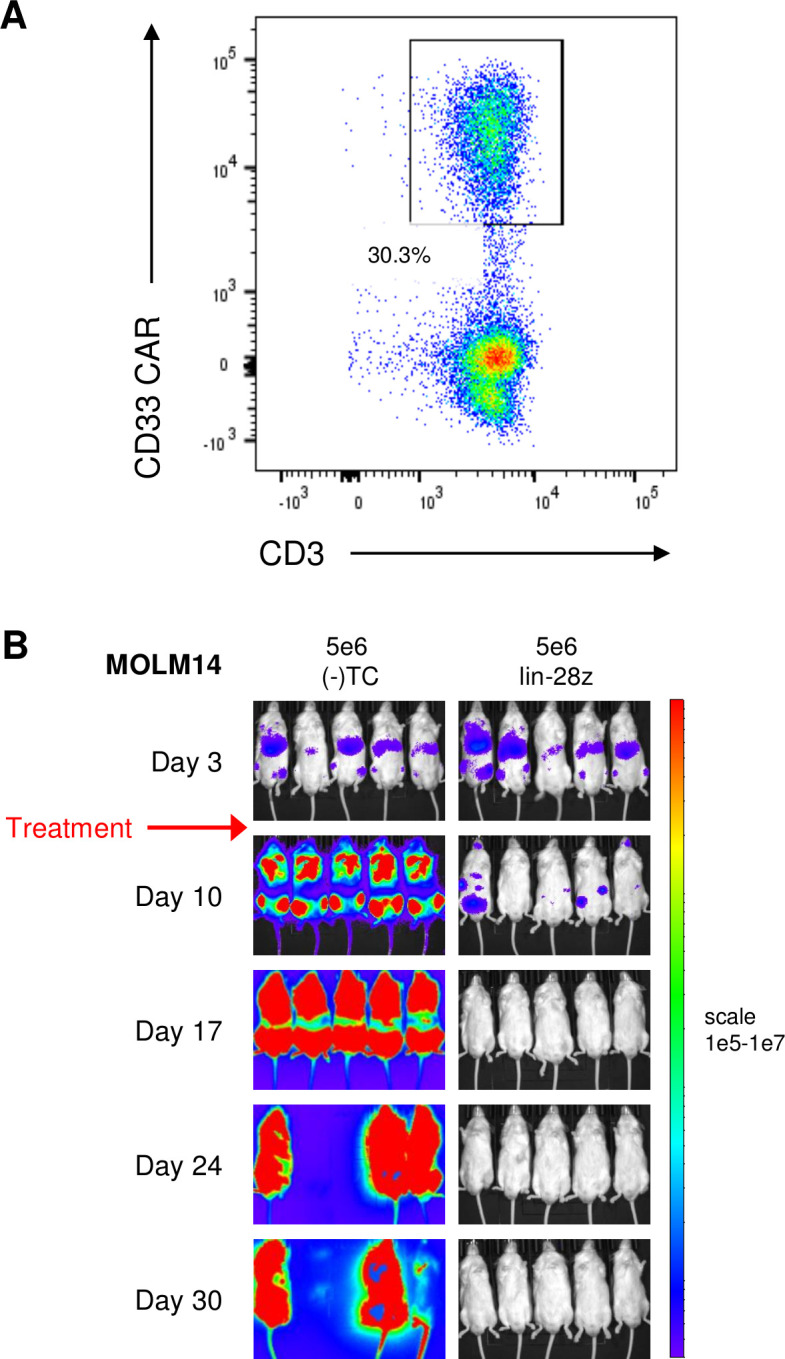
Preclinical validation of anti-leukemia activity of clinical-grade CD33 chimeric antigen receptor construct (CD33CART). The lintuzumab-based CD33CART with a CD28/CD3ζendodomain (lin-28z) was selected for further development and evaluation in a phase 1 clinical trial. (A) Transduction efficiency of clinical grade lin-28z CD33CART was 30.3% for this experiment. (B) Luciferase-transduced MOLM14 cells (1×106) were injected intravenous into NSG mice on day 0. Animals were assessed by bioluminescent imaging (BLI) and then randomized to treatment with GFP-transduced T cells ((-)TC) or clinical-grade lin-28z CD33CART cells (5×106 total cells/mouse) on day 3 (red arrow). Mice were followed by weekly BLI and euthanized when a predetermined maximal radiance level of 1×1010 photons/s/cm2/sr was detected Complete inhibition of leukemia proliferation was again observed with CD33CART.
