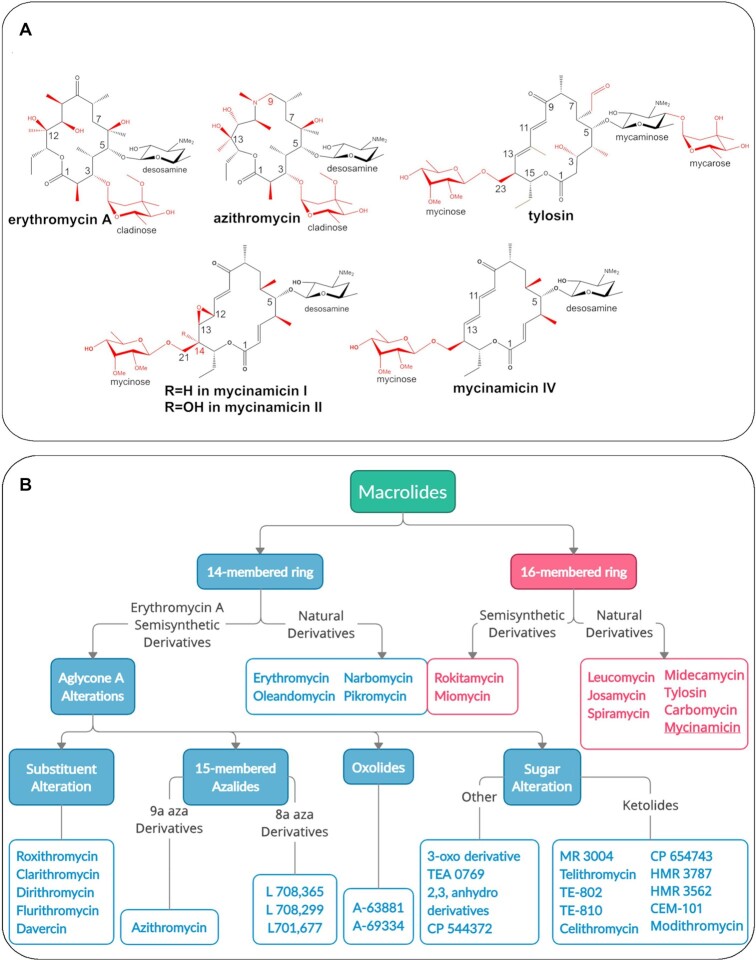
Figure 1.
(A) Chemical structures of macrolide antibiotics: natural product (first generation) erythromycin A contains a 14-membered macrolactone, second generation azithromycin includes a 15-membered aza-macrolactone ring, third generation tylosin, compared to mycinamicin I, II (also called mirosamycin), and IV. Chemical variations among the antibiotics are indicated in red. (B) Classification of natural and semisynthetic macrolides based on macrolactone ring size and chemical modifications (15) shows that the majority of clinical agents approved or under development comprise 14-membered macrolides and their derivatives.