Figure 1.
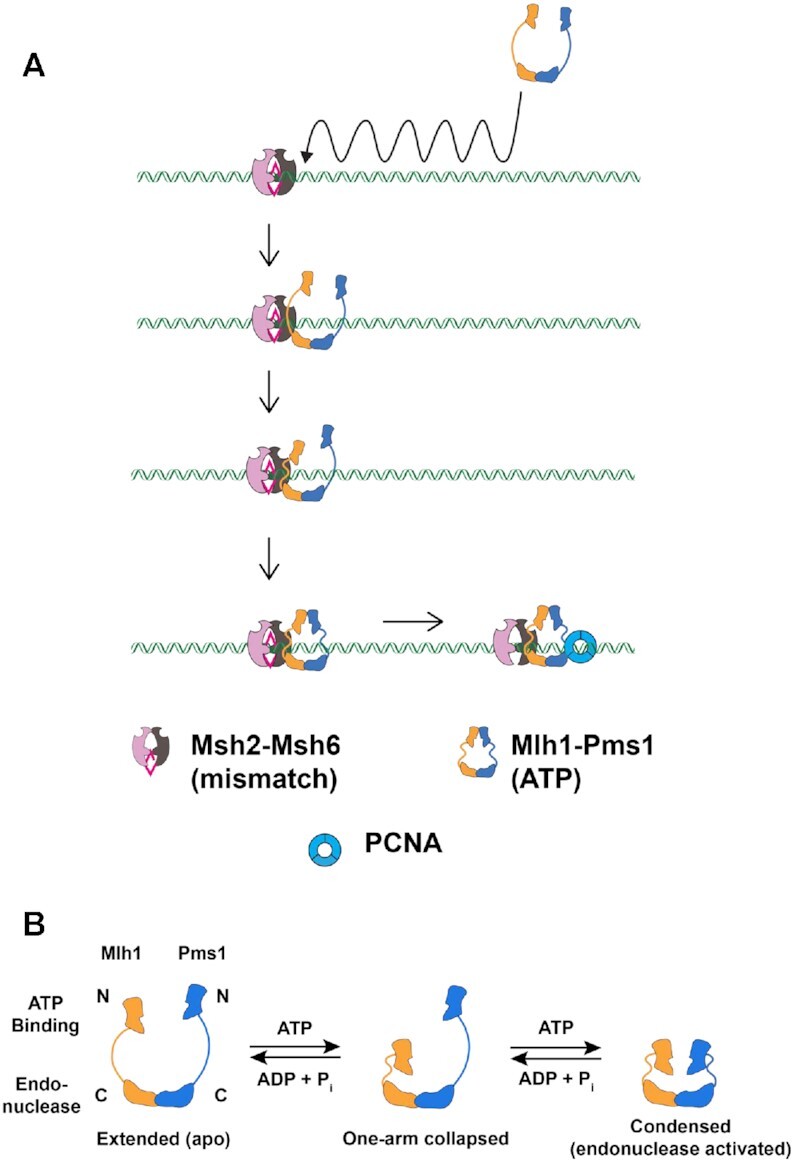
ATP-driven conformational changes in Mlh1–Pms1 during MMR. (A) A model for Mlh1–Pms1 interactions with MSH proteins during MMR. See Introduction for details. (B) Sacho et al. (21) proposed that ATP binding to one subunit of the MLH heterodimer promotes the formation of a one-armed collapsed state. Subsequent binding to the second subunit condenses its linker arm to yield a condensed complex that is thought to represent the activated endonuclease state.
