Abstract
Background.
In the phase 3 trial ARIEL3, maintenance treatment with the poly(ADP-ribose) polymerase (PARP) inhibitor rucaparib provided clinical benefit versus placebo for patients with recurrent, platinum-sensitive ovarian cancer. Here, we evaluate the impact of age on the clinical utility of rucaparib in ARIEL3.
Methods.
Patients with platinum-sensitive, recurrent ovarian carcinoma with ≥2 prior platinum-based chemotherapies who responded to their last platinum-based therapy were enrolled in ARIEL3 and randomized 2:1 to rucaparib 600 mg twice daily or placebo. Exploratory, post hoc analyses of progression-free survival (PFS), patient-centered outcomes (quality-adjusted PFS [QA-PFS] and quality-adjusted time without symptoms or toxicity [Q-TWiST]), and safety were conducted in three age subgroups (<65 years, 65–74 years, and ≥75 years).
Results.
Investigator-assessed PFS was significantly longer with rucaparib than placebo in patients aged <65 years (rucaparib n = 237 vs placebo n = 117; median, 11.1 vs 5.4 months; hazard ratio [HR]: 0.33 [95% confidence interval (95% CI) 0.25–0.43]; P < 0.0001) and 65–74 years (n = 113 vs n = 64; median, 8.3 vs 5.3 months; HR 0.43 [95% CI 0.29–0.63]; P < 0.0001) and numerically longer in patients aged ≥75 years (n = 25 vs n = 8; median, 9.2 vs 5.5 months; HR 0.47 [95% CI 0.16–1.35]; P = 0.1593). QA-PFS and Q-TWiST were significantly longer with rucaparib than placebo across all age subgroups. Safety of rucaparib was generally similar across the age subgroups.
Conclusions.
Efficacy, patient-centered outcomes, and safety of rucaparib were similar between age subgroups, indicating that all eligible women with recurrent ovarian cancer should be offered this therapeutic option, irrespective of age.
Keywords: Elderly patients, Maintenance, Ovarian cancer, PARP inhibitor, Rucaparib
1. Introduction
Ovarian cancer is a disease of the elderly, with older age associated with higher incidence and mortality rates than younger age [1,2]. The peak age range for a diagnosis of ovarian cancer is 55–64 years in the United States and 55–69 years in Europe, and estimated rates of new cases in 2018 were highest in women aged ≥70 years in both the United States and Europe [1,2]. In 2011, ovarian cancer-related mortality rates among women in Europe aged 70–79 years (38.69/100,000) were higher than rates in women aged 50–69 years (18.09/100,000) and 20–49 years (1.59/100,000). Similarly, in the United States in 2012, mortality was higher in women aged 70–79 years (36.51/100,000) than in those aged 50–69 years (17.30/100,000) and 20–49 years (1.23/100,000) [3]. Mortality due to ovarian cancer has fallen in developed countries over the past decade, but this decline was not evenly distributed across the age spectrum; mortality in younger women decreased by 21.7% over this period, but only declined by 2.2% in elderly women [4].
Despite higher disease incidence and mortality rates, older women with ovarian cancer tend to be underrepresented in clinical trials, and very little data are available about the optimal treatment for this population [4,5]. Older women with ovarian cancer are also less likely to receive surgery with or without chemotherapy than younger women [4]. The limited clinical evidence and a lack of confidence among physicians regarding their ability to manage elderly women with ovarian cancer may account for undertreatment, while poorer performance status, lower chemotherapy completion rates, and increased toxicities from chemotherapy and cytoreductive surgery in older patients [4,6,7] may also influence decision-making away from surgery and standard chemotherapies. Older women show increased toxicities to frontline chemotherapy compared with younger women, including neutropenia and neuropathy, and higher rates of neuropathy with paclitaxel chemotherapy for recurrent disease [6]; severe hematologic and gastroenterologic toxicities and neutropenia are also common in elderly patients receiving chemotherapy [4]. Ideally, treatment plans for older patients should be based on their frailty score, rather than their chronological age [4,6,7].
The global standard of care for advanced ovarian cancer is platinum- and taxane-based chemotherapy following cytoreductive surgery with or without interval debulking [8-10]. Most women will have disease recurrence and require additional therapies [11]. Targeted therapies such as antivascular endothelial growth factor therapy (bevacizumab) and poly (ADP-ribose) polymerase (PARP) inhibitors (rucaparib, olaparib, and niraparib) are now considered standard of care as second-line maintenance treatment [8,10,12], and olaparib (for patients with a BRCA1 or BRCA2 [BRCA] mutation) and bevacizumab are approved as maintenance treatment following frontline chemotherapy [13,14]. The goals of targeted maintenance treatment are to maintain the response achieved with chemotherapy and prolong the disease-free interval before recurrence [15], delaying the need for further chemotherapies and the associated toxicities that can negatively affect patients' quality of life (QoL) [16].
In the phase 3 ARIEL3 study (CO-338-014; NCT01968213) which enrolled patients with advanced, recurrent ovarian cancer, maintenance treatment with rucaparib significantly improved investigator-assessed and blinded independent central review (BICR)-assessed progression-free survival (PFS) versus placebo in all three molecularly defined, nested cohorts: patients with a BRCA-mutated carcinoma (germline, somatic, or unknown origin); patients with a homologous recombination deficient (HRD) carcinoma (BRCA mutation + BRCA wild-type and high loss of heterozygosity [LOH]); and the intent-to-treat (ITT) population [17]. Based on the results of ARIEL3, rucaparib has been approved in the United States and the European Union as monotherapy for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who have had a complete or partial response to platinum-based chemotherapy [18,19].
In the maintenance setting, the impact of treatment and associated adverse effects on the patient's QoL is an important consideration, particularly in elderly patients, as they are more vulnerable to chemotherapy-associated toxicities [6]. Patient-centered assessments that measure both quality and quantity of life, such as quality-adjusted progression-free survival (QA-PFS) and quality-adjusted time without symptoms or toxicity (Q-TWiST), are particularly relevant for targeted oncology therapies such as PARP inhibitors that are given continuously over extended durations of time [20].
To assess the impact of age on the clinical utility of rucaparib as maintenance treatment, we conducted post hoc exploratory analyses of efficacy (investigator- and BICR-assessed PFS), patient-centered outcomes (QA-PFS and Q-TWiST) and safety in three age subgroups (<65 years, 65–74 years and ≥75 years) of patients from ARIEL3.
2. Methods
2.1. Study design and patient population
This randomized, double-blind, multicenter, international, phase 3 trial was conducted at 87 hospitals and cancer centers in 11 countries. Patients were enrolled between April 7, 2014, and July 19, 2016. The study was approved by national or local institutional review boards and was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Council for on Harmonisation. Patients provided written informed consent before participation.
Patients were aged at least 18 years, had platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube carcinoma, had received at least two previous platinum-based chemotherapy regimens, and must have achieved either a complete response according to Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST) or a partial response defined either according to RECIST or as a serologic response based on Gynecologic Cancer Inter-Group cancer antigen 125 (CA-125) response criteria to their last platinum-based regimen. Full inclusion and exclusion criteria have been reported previously [17].
Patients were randomized 2:1 to rucaparib or placebo using a computer-generated system, with stratification according to homologous recombination repair gene mutation status (based on gene mutation only; mutation in BRCA1 or BRCA2, mutation in a non-BRCA gene associated with homologous recombination, or no mutation in BRCA or a homologous recombination gene); platinum-free interval following penultimate platinum-based regimen (6 to ≤12 months or >12 months); and best response to most recent platinum-based regimen (complete or partial response). Details of screening and blinding procedures that informed randomization stratifications have been described previously [17].
In ARIEL3, patients received oral rucaparib 600 mg twice daily or placebo in continuous 28-day cycles until disease progression (as assessed by RECIST), death, or other reason for discontinuation. Dose reductions (in decrements of 120 mg down to 240 mg) were permitted if a patient had a grade ≥3 or a persistent grade 2 adverse event (AE); treatment was discontinued following toxicity-related treatment interruption of >14 consecutive days. Disease assessments were conducted at screening, every 12 weeks during treatment (and after treatment for patients who discontinued for reasons other than disease progression), following clinical symptoms, and at treatment discontinuation. Patients completed the EuroQol 5-dimensions-3 levels (EQ-5D-3L) questionnaire at screening, on day 1 of each treatment cycle, at the treatment discontinuation visit, and at the 28-day follow-up visit. Safety assessments included monitoring for treatment-emergent AEs (TEAEs) classified per the Medical Dictionary for Drug Regulatory Activities version 19.1 [21] and graded per the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 [22].
The primary endpoint of ARIEL3 was investigator-assessed PFS (defined as the time from randomization to investigator-assessed disease progression according to RECIST or death); secondary endpoints included BICR-assessed PFS and safety. QA-PFS and Q-TWiST were evaluated as post hoc analyses using utility values derived from the EQ-5D-3L [23]. The results for the primary and secondary efficacy endpoints and patient-centered outcomes have been previously published for the three molecularly defined, nested cohorts [17,23]. Here we report exploratory post hoc age subgroup analyses of investigator- and BICR-assessed PFS, QA-PFS and Q-TWiST, and safety.
2.2. Statistical analysis
The rationale for the sample size (N = 564) has been reported previously [17]. Data were analyzed for three age subgroups (<65 years, 65–74 years, and ≥75 years) at the request of the Committee for Medicinal Products for Human Use (CHMP), in particular to characterize the safety of rucaparib in elderly patients.
Kaplan-Meier methodology was used to summarize PFS; patients without documented progression were censored as of their last tumor assessment. A stratified log rank test that included the randomization strata was used to compare treatments. A stratified Cox proportional hazard model was used to calculate the HR between the treatment groups for PFS. PFS endpoints were tested at a one-sided 0.025 significance level, without any multiplicity adjustment.
For QA-PFS and Q-TWiST analyses, the EQ-5D-3L index score was calculated using the UK value set obtained using time-trade-off methodology. QA-PFS was calculated as the product of the investigator-assessed PFS function and the EQ-5D-3L index score function. The PFS function until the visit cutoff date (April 15, 2017) was obtained by Kaplan-Meier estimation. The EQ-5D-3L index score function was obtained by computation of the mean EQ-5D-3L index score of patients who were alive and uncensored at each visit scheduled in the double-blind treatment period. To create a QoL function over continuous time, estimates of the mean EQ-5D-3L index score at each visit were connected, assuming a linear change. Mean QA-PFS was obtained by computing the area under the quality-survival product function. The 95% confidence interval (CI) for the mean QA-PFS in the rucaparib and placebo groups and for the difference between groups was computed using the bootstrap method [24], with 200 replications of the sample.
Mean time without toxicity or symptoms of disease progression (TWiST state) was calculated as the mean PFS time minus the mean time with toxicities (TOX state). Mean time with symptoms of disease (REL state) is typically calculated as the mean overall survival (OS) time minus the mean PFS time. As the OS data for ARIEL3 were not mature at the time of this analysis, this health state was not included.
Q-TWiST was calculated as μTOX × TOX + TWiST, where μTOX denotes the utility weight for the TOX state. The mean duration for the TOX state and the mean PFS time were estimated by the area under each survival curve and calculated using Kaplan-Meier estimates. Utility weight for the TWiST state was set to one (highest possible), as this state is the best state for patients in the clinical trial. Further details of μTOX determinations have been described previously [23].
For each patient, time with toxicity of treatment was defined as the number of days with grade ≥3 TEAEs after randomization and before disease progression or censoring for progression. All grade ≥3 TEAEs before progression were included in the calculation of time with toxicity of treatment. If several AEs overlapped, the number of days was calculated between the start date of the first AE and the end date of the last AE. An additional analysis was conducted in which time with toxicity of treatment was defined using grade ≥2 TEAEs of nausea, vomiting, fatigue, and asthenia only, as these TEAEs are frequently observed with rucaparib and other PARP inhibitors. Consistent with other TWiST analyses of PARP inhibitors as maintenance treatment for recurrent ovarian cancer [25,26], grade 1 TEAEs were excluded from these analyses as it is difficult to derive proper utility scores for grade 1 TEAEs. Furthermore, utility scores calculated for grade 1 TEAEs were expected to be low and unlikely to impact these analyses.
For patient-centered outcomes, 95% CIs were calculated using a two-sided bootstrap method. No method to control for multiple testing was applied because these were exploratory post hoc analyses.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patients
The visit cutoff dates were April 15, 2017, for efficacy and patient-centered outcomes and December 31, 2017, for safety analyses. A total of 375 patients were randomized to rucaparib and 189 to placebo. Baseline patient demographics, disease characteristics, and prior therapies in the ITT population for each age subgroup were well balanced between treatment arms, although a greater proportion of patients aged ≥75 years had poorer ECOG performance status (i.e., ≥1) than those aged <75 years. The proportion with BRCA mutations was greater in patients aged <65 years versus those aged ≥65 years (Table 1).
Table 1.
Baseline patient demographics, disease characteristics, and prior therapies according to age subgroup.
| Age <65 years |
Age 65–74 years |
Age ≥75 years |
||||
|---|---|---|---|---|---|---|
| Rucaparib (n = 237) |
Placebo (n = 117) |
Rucaparib (n = 113) |
Placebo (n = 64) |
Rucaparib (n = 25) |
Placebo (n = 8) |
|
| Age, median (range) | 56.0 (39.0–64.0) | 55.0 (36.0–64.0) | 68.0 (65.0–74.0) | 69.0 (65.0–74.0) | 76.0 (75.0–84.0) | 77.5 (76.0–85.0) |
| ECOG performance status, n (%) | ||||||
| 0 | 189 (79.7) | 88 (75.2) | 77 (68.1) | 44 (68.8) | 14 (56.0) | 4 (50.0) |
| 1 | 48 (20.3) | 29 (24.8) | 36 (31.9) | 20 (31.3) | 11 (44.0) | 4 (50.0) |
| Diagnosis, n (%) | ||||||
| Epithelial ovarian cancer | 205 (86.5) | 103 (88.0) | 87 (77.0) | 50 (78.1) | 20 (80.0) | 6 (75.0) |
| Fallopian tube cancer | 13 (5.5) | 4 (3.4) | 15 (13.3) | 6 (9.4) | 4 (16.0) | 0 |
| Primary peritoneal cancer | 19 (8.0) | 10 (8.5) | 11 (9.7) | 7 (10.9) | 1 (4.0) | 2 (25.0) |
| High-grade serous adenocarcinoma | 0 | 0 | 0 | 1 (1.6)a | 0 | 0 |
| BRCA mutation in carcinoma, n (%) | ||||||
| BRCA mutant | 96 (40.5) | 49 (41.9) | 29 (25.7) | 15 (23.4) | 5 (20.0) | 2 (25.0) |
| BRCA1 | 66 (27.8) | 31 (26.5) | 14 (12.4) | 5 (7.8) | 0 | 1 (12.5) |
| BRCA2 | 30 (12.7) | 18 (15.4) | 15 (13.3) | 10 (15.6) | 5 (20.0) | 1 (12.5) |
| Germline | 65 (27.4) | 38 (32.5) | 15 (13.3) | 8 (12.5) | 2 (8.0) | 2 (25.0) |
| Somatic | 25 (10.5) | 9 (7.7) | 12 (10.6) | 7 (10.9) | 3 (12.0) | 0 |
| BRCA unknownb | 6 (2.5) | 2 (1.7) | 2 (1.8) | 0 | 0 | 0 |
| BRCA wild-type | ||||||
| LOH high | 67 (28.3) | 29 (24.8) | 35 (31.0) | 21 (32.8) | 4 (16.0) | 2 (25.0) |
| LOH low | 57 (24.1) | 29 (24.8) | 40 (35.4) | 22 (34.4) | 10 (40.0) | 3 (37.5) |
| LOH indeterminatec | 17 (7.2) | 10 (8.5) | 9 (8.0) | 6 (9.4) | 6 (24.0) | 1 (12.5) |
| No. of prior chemotherapy regimens, median (range) | 2.0 (2.0–6.0) | 2.0 (2.0–5.0) | 2.0 (2.0–6.0) | 2.0 (2.0–6.0) | 2.0 (2.0–5.0) | 2.5 (2.0–4.0) |
| 2, n (%) | 149 (62.9) | 81 (69.2) | 68 (60.2) | 39 (60.9) | 14 (56.0) | 4 (50.0) |
| 3, n (%) | 63 (26.6) | 25 (21.4) | 37 (32.7) | 14 (21.9) | 8 (32.0) | 3 (37.5) |
| ≥4, n (%) | 25 (10.5) | 11 (9.4) | 8 (7.1) | 11 (17.2) | 3 (12.0) | 1 (12.5) |
| No. of previous Pt-based regimens, median (range) | 2.0 (2.0–5.0) | 2.0 (2.0–4.0) | 2.0 (2.0–6.0) | 2.0 (2.0–5.0) | 2.0 (2.0–4.0) | 2.5 (2.0–4.0) |
| 2, n (%) | 153 (64.6) | 82 (70.1) | 69 (61.1) | 40 (62.5) | 14 (56.0) | 4 (50.0) |
| ≥3, n (%) | 84 (35.4) | 35 (29.9) | 44 (38.9) | 24 (37.5) | 11 (44.0) | 4 (50.0) |
| TTP with penultimate Pt-based regimen, median (range) | 13.6 (6.0–115.4) | 16.9 (6.0–107.6) | 14.2 (5.8–120.0) | 12.3 (6.4–238.5) | 10.7 (6.1–106.7) | 13.3 (6.6–33.5) |
| 6 to ≤12, n (%) | 96 (40.5) | 38 (32.5) | 42 (37.2) | 34 (53.1) | 13 (52.0) | 4 (50.0) |
| >12, n (%) | 141 (59.5) | 79 (67.5) | 71 (62.8) | 30 (46.9) | 12 (48.0) | 4 (50.0) |
| Response to last Pt-based regimen, n (%) | ||||||
| CR according to RECIST | 93 (39.2) | 42 (35.9) | 26 (23.0) | 20 (31.3) | 7 (28.0) | 2 (25.0) |
| PR according to RECIST or serologic response according to GCIG CA-125 criteria | 144 (60.8) | 75 (64.1) | 87 (77.0) | 44 (68.8) | 18 (72.0) | 6 (75.0) |
CA-125, cancer antigen 125; CR, complete response; ECOG, Eastern Cooperative Oncology Group; GCIG, Gynecologic Cancer InterGroup; HRR, homologous recombination repair; LOH, loss of heterozygosity; PR, partial response; Pt, platinum; RECIST, Response Evaluation Criteria In Solid Tumors; TTP, time to progression.
According to the patient's records, origin was fallopian tube or ovary.
Tumor sample was BRCA mutant according to Foundation Medicine's T5 next-generation sequencing assay, but a blood sample was not available for central germline testing.
Tumor sample was not evaluable for percentage of genomic LOH because of low tumor content or aneuploidy.
3.2. Efficacy
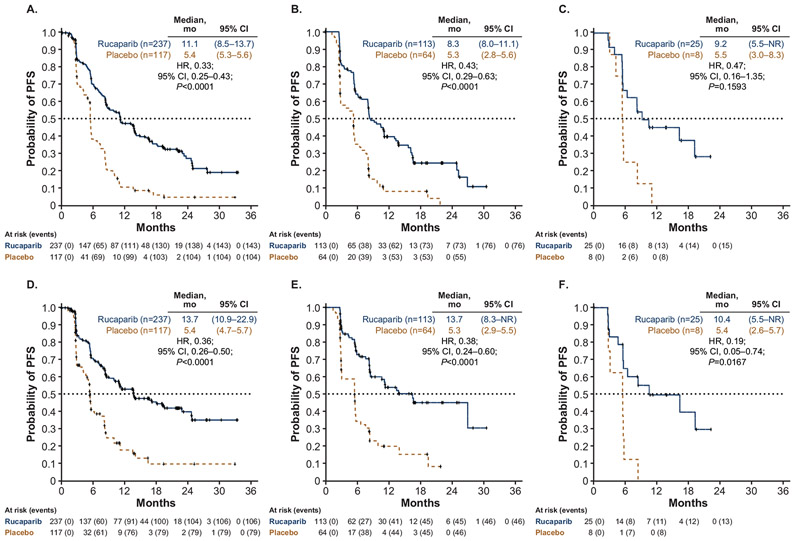
Median investigator-assessed PFS was significantly longer with rucaparib than placebo in the <65 years and 65–74 years subgroups, and numerically longer in the ≥75 years subgroup. For patients aged <65 years, median PFS was 11.1 months in the rucaparib arm (n = 237) versus 5.4 months in the placebo arm (n = 117) (hazard ratio [HR], 0.33; 95% CI, 0.25–0.43, P < 0.0001) (Fig. 1A); for patients aged 65–74 years, median PFS was 8.3 months (n = 113) versus 5.3 months (n = 64) (HR, 0.43; 95% CI, 0.29–0.63, P < 0.0001) (Fig. 1B); and for patients aged ≥75 years, median PFS was 9.2 months (n = 25) versus 5.5 months (n = 8) (HR, 0.47; 95% CI, 0.16–1.35, P = 0.1593) (Fig. 1C). Median BICR-assessed PFS was significantly longer in patients treated with rucaparib than those who received placebo in all age subgroups (Fig. 1D-F).
Fig. 1.
Probability of PFS as assessed by investigator (A, B, C) and BICR (D, E, F) according to age subgroup (A, D: <65 years; B, E: 65–74 years; C, F: ≥75 years). BICR, blinded independent central review; CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
3.3. Patient-centered outcomes
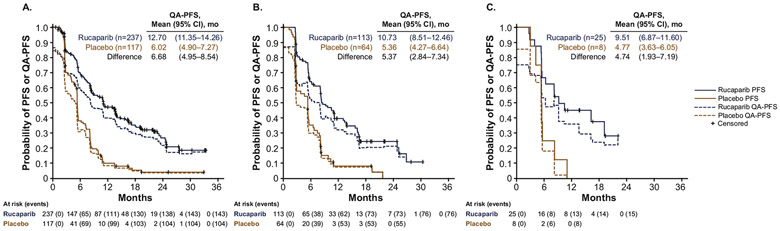
In the ITT population, mean QA-PFS was significantly longer in patients treated with rucaparib than those treated with placebo in all age subgroups, with similar mean differences between rucaparib and placebo across subgroups. In patients aged ≤65 years, mean (95% CI) QA-PFS was 12.70 (11.35–14.26) months in the rucaparib group versus 6.02 (4.90–7.27) months in the placebo group, with a mean (95% CI) difference of 6.68 (4.95–8.54) months (Fig. 2A). Findings were similar in patients aged 65–74 years with mean (95% CI) QA-PFS of 10.73 (8.51–12.46) months with rucaparib versus 5.36 (4.27–6.64) months with placebo (mean [95% CI] difference, 5.37 [2.84–7.34] months) and in patients aged ≥75 years with mean (95% CI) QA-PFS of 9.51 (6.87–11.06) versus 4.77 (3.63–6.05) months, respectively (mean [95% CI] difference, 4.74 [1.93–7.19] months) (Fig. 2B and C).
Fig. 2.
Comparison of investigator-assessed PFS and QA-PFS according to age subgroup (A: <65 years; B: 65–74 years; C: ≥75 years). CI, confidence interval; PFS, progression-free survival; QA-PFS, quality-adjusted progression-free survival.
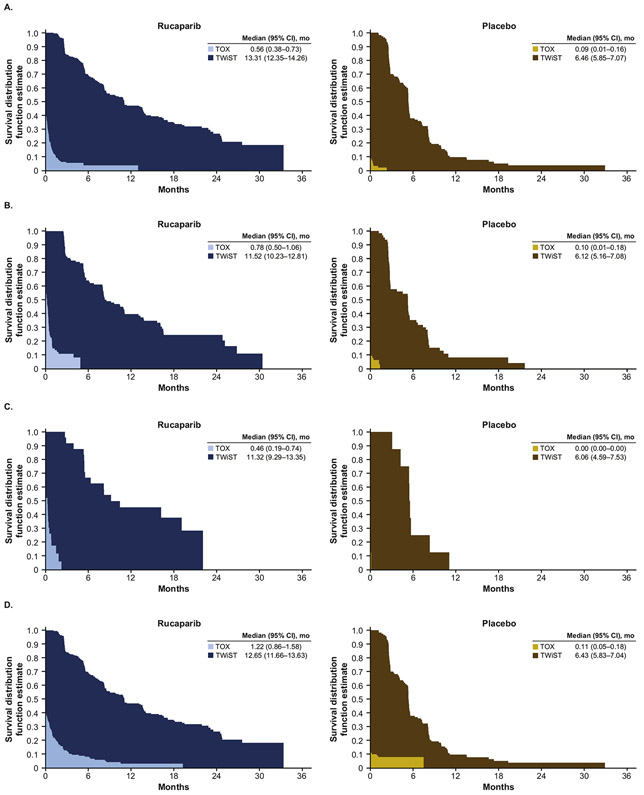
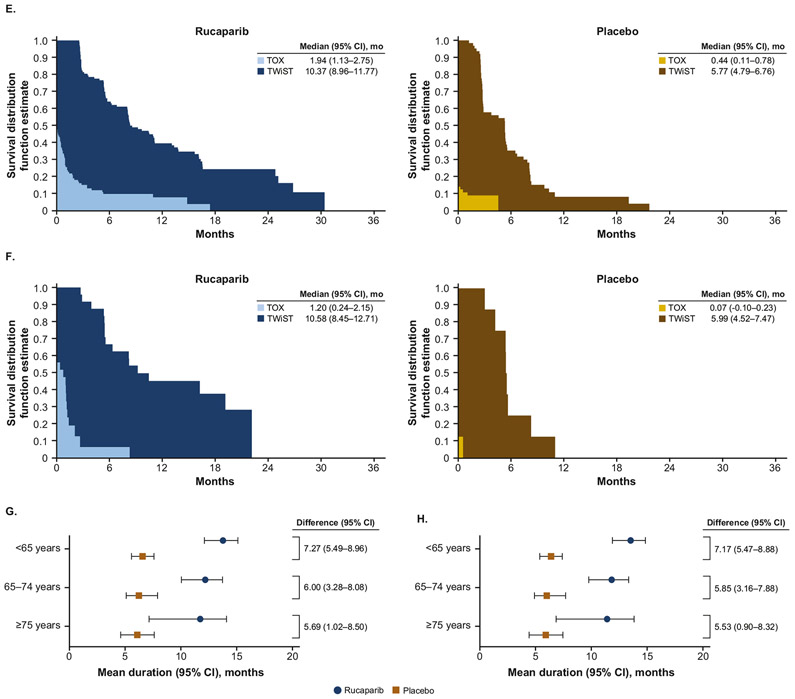
Mean duration with grade ≥3 TEAEs (TOX state) was longer with rucaparib than placebo in all age subgroups, with similar mean differences between rucaparib and placebo across the age subgroups (age <65 years: mean difference [95% CI], 0.47 [0.28–0.66] months; 65–74 years: 0.69 [0.39–0.98] months; ≥75 years, 0.46 [0.19–0.74] months). Despite these findings, mean TWiST was significantly longer with rucaparib than placebo in all age subgroups (age <65 years: mean difference [95% CI], 6.85 [5.72–7.98] months; 65–74 years: 5.40 [3.80–7.00] months; ≥75 years, 5.26 [2.88–7.63] months) demonstrating the clinical benefit of rucaparib over placebo despite its toxicity (Fig. 3A-C and Supplementary Table S1). In the quality-adjusted analyses, Q-TWiST was also longer with rucaparib than placebo in all age subgroups. Differences (95% CI) in mean Q-TWiST between rucaparib and placebo were 7.27 (5.49–8.96) months in patients aged <65 years, 6.00 (3.28–8.08) months in patients aged 65–74 years, and 5.69 (1.02–8.50) months in patients aged ≥75 years (Fig. 3G).
Fig. 3.
TWiST analyses with TOX defined as all grade ≥3 TEAEs (A: <65 years; B: 65–74 years; C: ≥75 years) and TOX defined as grade ≥2 TEAEs of nausea, vomiting, fatigue, and asthenia (D: <65 years; E: 65–74 years; F: ≥75 years), and Q-TWiST analyses by age subgroup with TOX defined as all grade ≥3 TEAEsa (G) and as grade ≥2 TEAEs of nausea, vomiting, fatigue, and astheniab (H). aμTOX values: 0.90 (<65 years), 0.87 (65–74 years), 0.94 (≥75 years). bμTOX values: 0.86 (<65 years), 0.84 (65–74 years), 0.83 (≥75 years). μTOX, mean utility weight for TOX health state; CI, confidence interval; Q-TWiST, quality-adjusted time without symptoms or toxicity; TEAE, treatment-emergent adverse event; TOX, time with toxicity of treatment.
When defining the TOX state using selected grade ≥2 TEAEs of nausea, vomiting, fatigue, and asthenia, duration of the TOX state, TWiST, and Q-TWiST were all longer with rucaparib than placebo in all age subgroups. Again, despite significantly longer mean TOX state duration with rucaparib than placebo in each age subgroup (age <65 years: mean difference [95% CI], 1.10 [0.74–1.47] months; 65–74 years: 1.50 [0.62–2.37] months; ≥75 years, 1.13 [0.16–2.09] months), TWiST was significantly longer with rucaparib than placebo in all age subgroups (age <65 years: mean difference [95% CI], 6.21 [5.06–7.37] months; 65–74 years: 4.59 [2.89–6.29] months; ≥75 years, 4.59 [2.13–7.05] months) (Fig. 3D-F and Supplementary Table S1). Q-TWiST was also significantly longer with rucaparib than placebo across age subgroups (age <65 years: mean difference [95% CI], 7.17 [5.47–8.88] months; 65–74 years, 5.85 [3.16–7.88] months; ≥75 years, 5.53 [0.90–8.32] months) (Fig. 3H).
3.4. Safety
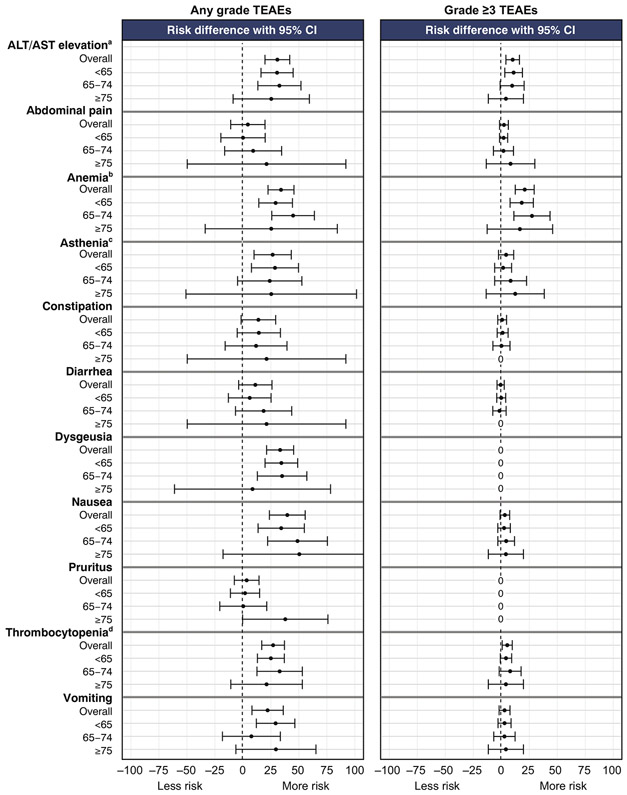
Almost all patients in the three age subgroups in both arms of the study reported one or more any-grade TEAE (Table 2). The most frequent (≥35% in any subgroup) any-grade TEAEs experienced by rucaparib-treated patients were nausea, asthenia/fatigue, vomiting, dysgeusia, constipation, anemia/decreased hemoglobin, AST/ALT elevations, diarrhea, abdominal pain, thrombocytopenia/decreased platelet count, and pruritus (Supplementary Table S2). In general, the relative risk of these AEs in each age subgroup was greater for rucaparib than placebo, although, compared with younger patients, CIs were wider in the age ≥75 years subgroup, likely driven by the lower number of patients in this subgroup (Fig. 4). The proportion of rucaparib-treated patients in each age group who experienced grade ≥3 TEAEs was slightly higher in the older-age subgroups: 127/235 (54.0%) in those aged <65 years, 79/113 (69.9%) in those aged 65–74 years, and 16/24 (66.7%) in those aged ≥75 years. The most frequent grade ≥3 TEAE with rucaparib in all age subgroups was anemia/decreased hemoglobin. The relative risk of grade ≥3 anemia/decreased hemoglobin and AST/ALT elevations appeared higher with rucaparib than placebo in all age subgroups (Fig. 4).
Table 2.
Summary of safety according to age subgroup.
| Age <65 years |
Age 65–74 years |
Age ≥75 years |
||||
|---|---|---|---|---|---|---|
| Rucaparib (n = 235)a |
Placebo (n = 117) |
Rucaparib (n = 113) |
Placebo (n = 64) |
Rucaparib (n = 24)a |
Placebo (n = 8) |
|
| Treatment duration, median (range), mo | 8.7 (0.1–43.4) | 5.5 (1.2–43.9) | 6.4 (0.2–38.1) | 5.2 (0.0–23.0) | 8.7 (2.9–32.8) | 5.4 (3.3–11.0) |
| Any-grade TEAE, n (%) | 235 (100.0) | 112 (95.7) | 113 (100.0) | 62 (96.9) | 24 (100.0) | 8 (100.0) |
| Grade ≥3 TEAE | 127 (54.0) | 19 (16.2) | 79 (69.9) | 10 (15.6) | 16 (66.7) | 1 (12.5) |
| Treatment interruption and/or dose reduction due to TEAE, n (%) | 154 (65.5) | 11 (9.4) | 93 (82.3) | 8 (12.5) | 20 (83.3) | 1 (12.5) |
| Treatment interruption due to TEAE | 141 (60.0) | 10 (8.5) | 83 (73.5) | 8 (12.5) | 19 (79.2) | 1 (12.5) |
| Dose reduction due to TEAE | 110 (46.8) | 3 (2.6) | 80 (70.8) | 4 (6.3) | 16 (66.7) | 1 (12.5) |
| Discontinued due to TEAE,b n (%) | 28 (11.9) | 2 (1.7) | 24 (21.2) | 1 (1.6) | 5 (20.8) | 0 |
| Deaths due to TEAE, n (%) | 5c (2.1) | 0 | 1d (0.9) | 2e (3.1) | 1f (4.2) | 0 |
| Deaths due to disease progression | 2 (0.9) | 0 | 0 | 1 (1.6) | 0 | 0 |
TEAE, treatment-emergent adverse event.
Three patients randomized to the rucaparib group (age <65 years, n = 2; age 75 years, n = 1) did not receive a dose of rucaparib and are excluded from the safety population.
Excluding disease progression.
Non–disease progression TEAEs leading to death: acute myeloid leukemia, n = 1; cardiac arrest, n = 1; myelodysplastic syndrome, n = 1.
Non–disease progression TEAE leading to death: hematophagic histiocytosis.
Non–disease progression TEAE leading to death: pulmonary embolism.
Non–disease progression TEAE leading to death: high grade B-cell lymphoma.
Fig. 4.
Forest plot of relative risk of most frequent any grade TEAEs (≥35% of patients. in any age subgroup) and grade ≥3 TEAEs. aCombined AST elevation and ALT elevation. bCombined anemia and decreased hemoglobin. cCombined asthenia and fatigue. dCombined thrombocytopenia and decreased platelet count. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; TEAEs, treatment-emergent adverse events.
In the rucaparib arm, dose modifications (treatment interruptions and/or dose reductions) and treatment discontinuations tended to be higher in patients aged ≥65 years than in patients aged <65 years. Rucaparib dose modification due to a TEAE occurred in 154/235 (65.5%) patients aged <65 years; 93/113 (82.3%) aged 65–74 years; and 20/24 (83.3%) aged ≥75 years. Discontinuation of rucaparib due to a TEAE occurred in 28/235 (11.9%) of patients aged <65 years, 24/113 (21.2%) of patients aged 65–74 years, and 5/24 (20.8%) of patients aged ≥75 years.
Deaths due to TEAEs in patients treated with rucaparib were reported at similar levels in all age subgroups: 5/235 (2.1%) aged <65 years; 1/113 (0.9%) aged 65–74 years; and 1/24 (4.2%) aged ≥75 years (Table 2). In the placebo arm, deaths due to TEAEs were reported in 0/117 (0%), 2/64 (3.1%) and 0/8 (0%) patients, respectively. Deaths due to progressive disease occurred in 2/235 (0.9%) patients aged <65 years who had been treated with rucaparib, and 1/64 (1.6%) patients aged 65–74 years who had been treated with placebo; no deaths due to progressive disease were reported in patients treated with rucaparib aged ≥75 years or in patients treated with placebo aged <65 years or ≥75 years.
4. Discussion
Our analyses demonstrate that irrespective of patient age, rucaparib maintenance treatment improved PFS and patient-centered outcomes compared with placebo. The safety profile was also similar across age groups. These findings build on the primary efficacy findings of ARIEL3, which showed that maintenance treatment with PARP inhibitors such as rucaparib should be considered a new standard of care for women with platinum-sensitive ovarian cancer following a complete or partial response to second- or later-line platinum-based chemotherapy [17].
In the overall ITT population from ARIEL 3, rucaparib maintenance treatment significantly extended median investigator-assessed PFS versus placebo (mean [95% CI] 10.8 [95% CI, 8.3–11.4] months versus 5.4 [5.3–5.5] months, respectively; HR [95% CI], 0.36 [0.30–0.45]; P < 0.0001) [17]. ARIEL3 enrolled patients across a wide range of ages, with 37.2% of patients aged ≥65 years. Here we show that rucaparib provided longer investigator-assessed PFS than placebo in all age subgroups investigated (<65 years, 65–74 years, ≥75 years), with median duration of improvement and reduced risk of progression in each age subgroup that were similar to those of the ITT population. These findings are supported by analyses of BICR-assessed PFS, which was significantly longer with rucaparib across all age subgroups. Although a lower proportion of patients aged ≥65 years had a tumor with a BRCA mutation compared with those aged <65 years, rucaparib demonstrated efficacy over placebo across all age subgroups.
In a previous analysis of ARIEL3 data, the PFS benefit of rucaparib was shown to persist when adjusted by patients' perceptions of their health status [23]. In the overall ITT population, the mean (95% CI) difference in QA-PFS between the rucaparib and placebo groups was 6.3 (4.9–7.5) months. Here we found that rucaparib had a similar beneficial effect on QA-PFS in analyses conducted across age subgroups. Additionally, previous Q-TWiST analyses showed that rucaparib maintenance treatment extended the time during which patients had good health status or QoL without cancer-related symptoms or treatment toxicity. In the ARIEL3 ITT population, the mean (95% CI) difference in Q-TWiST (based on grade ≥3 TEAEs) between the rucaparib and placebo groups was 6.9 (5.7–8.2) months. The present analyses demonstrate that rucaparib was associated with longer Q-TWiST than placebo irrespective of age, despite longer time with TEAEs (in both grade ≥3 TEAE and selected grade ≥2 TEAE analyses). These findings are of particular importance in elderly patients since they suggest that even older women derive clinical benefit from rucaparib maintenance therapy despite the impact of prolonged toxicity times.
There were no differences between age subgroups in the proportion of patients with any-grade TEAEs, or in relative risk of specific any-grade TEAEs for rucaparib versus placebo. The proportion of patients experiencing grade ≥3 TEAEs was slightly higher in patients aged ≥65 years, but relative risk (rucaparib vs placebo) of specific grade ≥3 TEAEs were generally similar between the age subgroups, suggesting that the potentially greater severity of TEAEs in older patients should not discourage recommendation of rucaparib as a therapeutic option in older patients. Dose modification and treatment discontinuation rates were somewhat higher in patients aged ≥65 years but were not markedly greater than those observed in the overall safety population [17], and were not associated with increased mortality in any age subgroup. As with the overall population, older patients should be provided with supportive care to address any TEAEs and consider dose interruption or reduction if needed.
Subgroup analyses of the efficacy and safety of maintenance therapy with PARP inhibitors or bevacizumab in different age groups have been performed using data from various studies [27-29]. However, our analysis of the age-related ARIEL3 data is the first to report the impact of age on patient-centered outcomes (QA-PFS, Q-TWiST). In patients with recurrent, platinum-sensitive ovarian cancer and a germline BRCA mutation enrolled in the NOVA trial, niraparib was associated with a reduced risk of disease progression or death versus placebo in patients aged <70 years (n = 182; HR, 0.30 [95% CI, 0.19–0.47]) and aged ≥70 years (n = 21; HR, 0.09 [95% CI, 0.01–0.73]). Reduced risk of disease progression or death with niraparib was also observed in patients aged <70 years who did not have a germline BRCA mutation (n = 276; HR, 0.47 [95% CI, 0.34–0.66] and aged ≥70 years (n = 74; HR, 0.35 [95% CI, 0.18–0.71]) [29]. There was no difference in rates of hematologic toxicities between patients aged <70 years and patients aged ≥70 years. In an analysis of age subgroups from eight completed prospective trials of olaparib, there was no statistical difference in toxicity by age, but trends toward more hematologic toxicity with increasing age were reported [28]. In the OCEANS trial, the efficacy of bevacizumab maintenance therapy versus placebo added to gemcitabine chemotherapy as assessed by overall survival was not different between patients aged <65 years (n = 306; HR, 0.89 [95% CI, 0.69–1.16]) and patients aged ≥65 years (n = 178; HR, 1.06 [95% CI, 0.75–1.50]) [27].
Because the eligibility criteria for ARIEL3 were not overly restrictive, the enrolled population may be considered to resemble the real-world patient population. Limitations of the current analyses are that they were not prespecified and that ARIEL3 was not designed or powered to evaluate PFS or other outcomes in specific age subgroups. In particular, the small patient population aged ≥75 years precludes strong conclusions being drawn for this age group.
Overall, these findings demonstrate that the efficacy and safety profile associated with rucaparib maintenance treatment is similar in elderly and younger patients. The HR for PFS with rucaparib versus placebo was similar across all analyzed age groups. QA-PFS and Q-TWiST results, which incorporate patient-reported perspectives, were similar in elderly and younger patients, suggesting that rucaparib provided significant benefits to patient health status that were not affected by toxicities, irrespective of patient age. The safety profile of rucaparib was generally similar between age subgroups, despite older patients being reported to have a greater susceptibility to toxicities from standard chemotherapies [4,6]. Given the increasing numbers of elderly patients who may be eligible for maintenance treatment [4,12], these results should provide clinicians with reassurance that, provided an elderly woman is eligible for rucaparib treatment, there is no reason to withhold this therapeutic option.
Supplementary Material
HIGHLIGHTS.
PFS was significantly longer with rucaparib maintenance treatment vs placebo across age subgroups (<65, 65–74, and ≥75 y).
Rucaparib provided significant improvements in patient-centered outcomes (QA-PFS; Q-TWiST) vs placebo across age subgroups.
The safety profile of rucaparib was generally similar across age subgroups.
Acknowledgments
The study was funded by Clovis Oncology, Inc. Medical writing and editorial support were provided by Jeremy Kennard, PhD, and Frederique H. Evans, MBS, of Ashfield Healthcare Communications.
Role of the funding source
ARIEL3 was designed by Elizabeth M. Swisher, Jonathan A. Ledermann, and Robert L. Coleman in collaboration with the funder. This article was written by the authors, with medical writing and copyediting support paid for by the funder. Data were collected by the investigators, analyzed by the funder, and interpreted by all authors. All authors had full access to all trial data and had final responsibility for the decision to submit these data for publication.
Declaration of competing interest
Nicoletta Colombo: Served in a consulting or advisory role for Clovis Oncology, AstraZeneca, BIOCAD, Pfizer, PharmaMar, Roche, and Tesaro.
Amit M. Oza: Served on advisory boards for Clovis Oncology, Amgen, AstraZeneca, GlaxoSmithKline, and Immunovaccine.
Domenica Lorusso: Served in a consulting or advisory role for Clovis Oncology, AstraZeneca, ImmunoGen, Merck, PharmaMar, Roche, Takeda, and Tesaro and received support for travel or accommodation from PharmaMar and Roche.
Carol Aghajanian: Served on a steering committee for Clovis Oncology, AbbVie, Genentech, and Mateon Therapeutics; served on advisory boards for Clovis Oncology, Cerulean Pharma, Eisai/Merck, ImmunoGen, and Tesaro; received research grants from Clovis Oncology, AbbVie, AstraZeneca, and Genentech; and received honoraria from Clovis Oncology, Cerulean Pharma, Eisai/Merck, ImmunoGen, Mateon Therapeutics, and Tesaro.
Ana Oaknin: Served on advisory boards for Clovis Oncology, AstraZeneca, ImmunoGen, Genmab/Seattle Genetics, PharmaMar, Roche, and Tesaro; received support for travel or accommodation from Clovis Oncology, AstraZeneca, PharmaMar, and Roche; and received research grants from Clovis Oncology, AbbVie Deutschland, Ability Pharmaceuticals, Advaxis, Aeterna Zentaris, Amgen SA, Aprea Therapeutics AB, Bristol-Myers Squibb, Eisai, F. Hoffmann-La Roche, ImmunoGen, Merck Sharp & Dohme de España SA, Millennium Pharmaceuticals, PharmaMar, Regeneron Pharmaceuticals, and Tesaro.
Andrew Dean: Served in a consulting or advisory role for Precision Oncology Australia, Shire Pharmaceuticals, and Specialised Therapeutics Australia.
Johanne I. Weberpals: Received research support from AbbVie and AstraZeneca and served on advisory boards for AstraZeneca.
Andrew R. Clamp: Served on advisory boards for AstraZeneca; received research funding from Clovis Oncology and AstraZeneca; and received support for travel and accommodation for congress attendance from Clovis Oncology, AstraZeneca, and Roche.
Giovanni Scambia: Served in a consulting or advisory role for Clovis Oncology, AstraZeneca, PharmaMar, Roche, and Tesaro.
Alexandra Leary: Served on advisory boards for Clovis Oncology, AstraZeneca, BIOCAD, GamaMabs, Genmab/Seattle Genetics, Merck Sharp & Dohme, Pfizer, PharmaMar, and Tesaro; received support for travel and accommodation from Clovis Oncology, AstraZeneca, Roche, and Tesaro; and reports institutional research grant support from Clovis Oncology, AstraZeneca, GamaMabs, Inivata, Merck Sharp & Dohme, Merus, Sanofi, and Tesaro.
Robert W. Holloway: Served on speakers bureaus for Clovis Oncology, AstraZeneca, and Tesaro.
Margarita Amenedo Gancedo: Served on advisory boards for Clovis Oncology and on speakers bureaus for AstraZeneca, PharmaMar, and Roche.
Peter C. Fong: Served on advisory boards for Clovis Oncology and AstraZeneca and received honoraria from AstraZeneca.
Jeffrey C. Goh: Served in a consulting or advisory role for AstraZeneca, Bristol-Myers Squibb and Tesaro; served on speakers bureaus for Ipsen and Merck Sharp & Dohme; and received support for travel or accommodation from Astellas, AstraZeneca, and Bristol-Myers Squibb.
David M. O'Malley: Served on advisory boards for Clovis Oncology, AbbVie, AstraZeneca, Eisai, Genentech/Roche, Genelux, Iovance Biotherapeutics, Janssen, Novocure, Regeneron, and Tesaro; has served on steering committees for Clovis Oncology, Agenus, Amgen, and Novocure; has served as a consultant for AbbVie, Ambry, AstraZeneca, Genentech/Roche, Gynecologic Oncology Group Foundation, and Tesaro; has given a presentation on ovarian cancer at the National Comprehensive Cancer Network; and his institution has received research support from Clovis Oncology, AbbVie, Agenus, Amgen, Ajinomoto, Array BioPharma, AstraZeneca, Bristol-Myers Squibb, Cerulean Pharma, Eisai, EMD Serono, ERGOMED Clinical Research, Genentech, Gynecologic Oncology Group, INC Research, inVentiv Health Clinical, Iovance Biotherapeutics, Janssen Research and Development, Ludwig Institute for Cancer Research, New Mexico Cancer Care Alliance, Novocure, PRA International, Regeneron Pharmaceuticals, Serono, Stemcentrx, Tesaro, TRACON Pharmaceuticals, VentiRx, and Yale University.
Deborah K. Armstrong: Served as a scientific advisor for Morphotek and received research funding from Clovis Oncology, Advaxis, AstraZeneca, Pfizer, Syndax, and Tesaro.
Susana Banerjee: Served on advisory boards and received honoraria from Clovis Oncology, AstraZeneca, PharmaMar, Seattle Genetics, and Tesaro; received honoraria from Merck Serono and Roche; and received support for travel or accommodation from NuCana and Tesaro.
Jesus García-Donas: Received research funding from AstraZeneca, Pierre Fabre, and Pfizer; received personal fees from Clovis Oncology, Astellas, Pierre Fabre, and Pfizer; and received nonfinancial support from Astellas, Pierre Fabre, and Pfizer.
Elizabeth M. Swisher: Has nothing to disclose.
Juliette Meunier: Employee of Modus Outcomes, which was contracted by Clovis Oncology to conduct these analyses.
Terri Cameron, Lara Maloney, Sandra Goble, and Josh Bedel: Employees of Clovis Oncology and may own stock or have stock options in that company.
Jonathan A. Ledermann: Received lecture fees from Clovis Oncology, AstraZeneca, Merck/Merck Sharp & Dohme, Pfizer, and Tesaro; served on advisory boards for Clovis Oncology, Artios Pharma, AstraZeneca, Cristal Therapeutics, Merck/Merck Sharp & Dohme, Pfizer, Regeneron, Roche, Seattle Genetics, and Tesaro; and received research grants from AstraZeneca and Merck/Merck Sharp & Dohme.
Robert L. Coleman: Reports grants from Clovis Oncology, AstraZeneca, Gateway Foundation, Janssen, Judy Reis/Albert Pisani, MD, Ovarian Cancer Research Fund, Merck, National Institutes of Health, Roche/Genentech, and V-Foundation; has served as an advisor to Clovis Oncology, Agenus, AstraZeneca, GamaMabs, Genmab, Janssen, OncoQuest, Pfizer (Medivation), Regeneron, Roche/Genentech, and Tesaro; and has an endowment as the Ann Rife Cox Chair in Gynecology.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2020.05.045.
References
- [1].Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. , Global cancer observatory: cancer today, https://gco.iarc.fr/today2018. (Accessed date: 23 January 2020). [Google Scholar]
- [2].National Cancer Institute (NCI), SEER Cancer Statistics Factsheets: Ovarian Cancer. http://seer.cancer.gov/statfacts/html/ovary.html (Accessed date: 23 January 2020). [Google Scholar]
- [3].Malvezzi M, Carioli G, Rodriguez T, Negri E, La Vecchia C, Global trends and predictions in ovarian cancer mortality, Ann. Oncol 27 (2016) 2017–2025. [DOI] [PubMed] [Google Scholar]
- [4].Tortorella L, Vizzielli G, Fusco D, Cho WC, Bernabei R, Scambia G, et al. , Ovarian cancer management in the oldest old: improving outcomes and tailoring treatments, Aging Dis. 8 (2017) 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liposits G, Loh KP, Soto-Perez-de-Celis E, Dumas L, Battisti NML, Kadambi S, et al. , PARP inhibitors in older patients with ovarian and breast cancer: Young International Society of Geriatric Oncology review paper, J. Geriatr. Oncol 10 (2019) 337–345. [DOI] [PubMed] [Google Scholar]
- [6].Tew WP, Ovarian cancer in the older woman, J. Geriatr. Oncol 7 (2016) 354–361. [DOI] [PubMed] [Google Scholar]
- [7].Dumas L, Ring A, Butler J, Kalsi T, Harari D, Banerjee S, Improving outcomes for older women with gynaecological malignancies, Cancer Treat. Rev 50 (2016) 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. , ESMO–ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurent disease, Ann. Oncol 30 (2019) 672–705. [DOI] [PubMed] [Google Scholar]
- [9].Kemp Z, Ledermann JA, Update on first-line treatment of advanced ovarian carcinoma, Int. J. Womens Health 5 (2013) 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer (Version 3.2019), https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf2019. (Accessed date: 23 January 2020).
- [11].Bouberhan S, Pujade-Lauraine E, Cannistra SA, Advances in the management of platinum-sensitive relapsed ovarian cancer, J. Clin. Oncol 37 (2019) 2424–2436. [DOI] [PubMed] [Google Scholar]
- [12].Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. , Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up, Ann. Oncol 24 (Suppl. 6) (2013) vi24–vi32. [DOI] [PubMed] [Google Scholar]
- [13].Lynparza (Olaparib) Tablets [Prescribing Information], AstraZeneca Pharmaceuticals, Wilmington, DE, 2020. [Google Scholar]
- [14].Avastin (Bevacizumab) Injection, for Intravenous Use [Prescribing Information], Genentech, Inc., South San Francisco, CA, 2020. [Google Scholar]
- [15].DiSilvestro P, Alvarez Secord A, Maintenance treatment of recurrent ovarian cancer: is it ready for prime time? Cancer Treat. Rev 69 (2018) 53–65. [DOI] [PubMed] [Google Scholar]
- [16].Markman M, Maintenance chemotherapy in the management of epithelial ovarian cancer, Cancer Metastasis Rev. 34 (2015) 11–17. [DOI] [PubMed] [Google Scholar]
- [17].Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. , Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial, Lancet. 390 (2017) 1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rubraca (Rucaparib) Tablets [Prescribing Information], Clovis Oncology, Inc., Boulder, CO, 2020. [Google Scholar]
- [19].Rubraca (Rucaparib) Tablets [Summary of Product Characteristics], Clovis Oncology Ireland Ltd., Swords, Ireland, 2019. [Google Scholar]
- [20].Cabarrou B, Filleron T, Boher JM, Bogart E, Tresch-Bruneel E, Penel N, et al. , How to report toxicity associated with targeted therapies? Ann. Oncol 27 (2016) 1633–1638. [DOI] [PubMed] [Google Scholar]
- [21].Brown EG, Wood L, Wood S, The medical dictionary for regulatory activities (MedDRA), Drug Saf. 20 (1999) 109–117. [DOI] [PubMed] [Google Scholar]
- [22].National Cancer Institute, NCI Term Browser, CTCAE. https://nciterms.nci.nih.gov/ncitbrowser/pages/vocabulary.jsf?dictionary=CTCAE&version=4.03 (Accessed date: 23 January 2020). [Google Scholar]
- [23].Oza A, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, et al. , Patient-centered outcomes in ARIEL3, a phase 3, randomized, placebo-controlled trial of rucaparib maintenance treatment in patients with recurrent ovarian carcinoma, J. Clin. Oncol (2020) In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Greene WH, Econometric Analysis (Fifth Edition), Prentice Hall, Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- [25].Friedlander M, Gebski V, Gibbs E, Davies L, Bloomfield R, Hilpert F, et al. , Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial, Lancet Oncol. 19 (2018) 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Matulonis UA, Walder L, Nottrup TJ, Bessette P, Mahner S, Gil-Martin M, et al. , Niraparib maintenance treatment improves time without symptoms or toxicity (TWiST) versus routine surveillance in recurrent ovarian cancer: a TWiST analysis of the ENGOT-OV16/NOVA trial, J. Clin. Oncol 37 (2019) 3183–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV, Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer, Gynecol. Oncol 139 (2015) 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dockery LE, Tew WP, Ding K, Moore KN, Tolerance and toxicity of the PARP inhibitor olaparib in older women with epithelial ovarian cancer, Gynecol. Oncol 147 (2017) 509–513. [DOI] [PubMed] [Google Scholar]
- [29].Fabbro M, Moore KN, Dorum A, Tinker AV, Mahner S, Bover I, et al. , Efficacy and safety of niraparib as maintenance treatment in older patients (≥70 years) with recurrent ovarian cancer: results from the ENGOT-OV16/NOVA trial, Gynecol. Oncol 152 (2019) 560–567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.