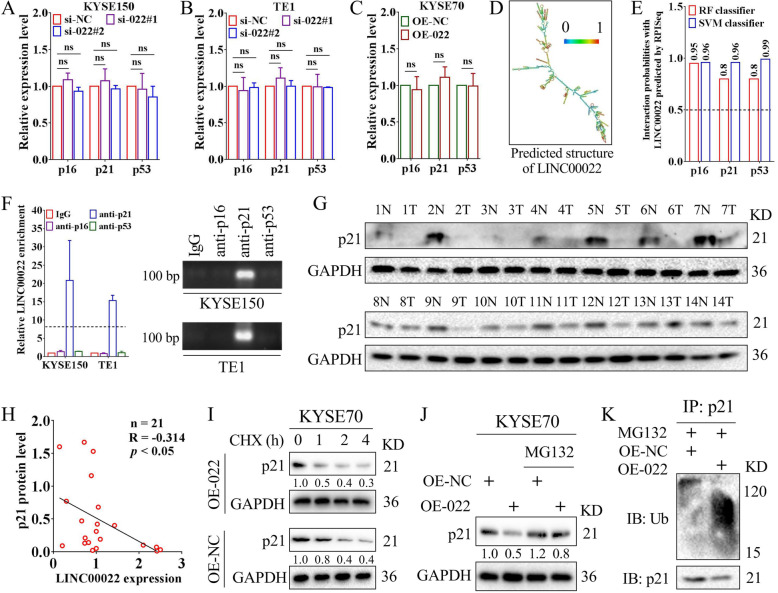
Fig. 5.
LINC00022 promotes ubiquitin-mediated p21 protein instability. (A-C) The mRNA levels of p16, p21 and p53 in KYSE150 (A) and TE1 (B) cells following LINC00022 knockdown and in KYSE70 (C) cells following LINC00022 over-expression were tested by qRT-PCR, ns means no significance. The mRNA levels of p16, p21 and p53 in ESCC cells were not significantly affected by either LINC00022 knockdown or LINC00022 over-expression. (D) The putative secondary structure of LINC00022 transcript in minimum free energy (MFE) mode was computational analyzed by RNAfold server. (E) The affinity of LINC00022 transcript with p16, p21, and p53 proteins was predicted by the machine learning classifier RPISeq based on Random Forest (RF) and Support Vector Machine (SVM) classifiers. (F) The direct binding affinity of LINC00022 transcript with p16, p21, or p53 proteins was determined by RIP-qRT-PCR (left panel) and agarose gel electrophoresis (right panel). (G) Western blot was used to examine the protein levels of p21 in 21 pairs of ESCC tissues, of which 14 pairs were shown. N represents normal and T represents tumor. (H) Person’s Coefficient analysis revealed the negative correlation between LINC00022 transcription and p21 protein in 21 cases of ESCC tumors. (I) Western blot was performed to observe the effect of LINC00022 over-expression on the stability of p21 protein in ESCC cells in the presence of protein synthesis inhibitor CHX (100 μg/mL). (J) The proteasome inhibitor MG132 (5 μM) partially relieved the instability of p21 protein caused by LINC00022 over-expression. (K) Co-IP combined with Western blot revealed the activated ubiquitination of p21 protein induced by LINC00022 over-expression