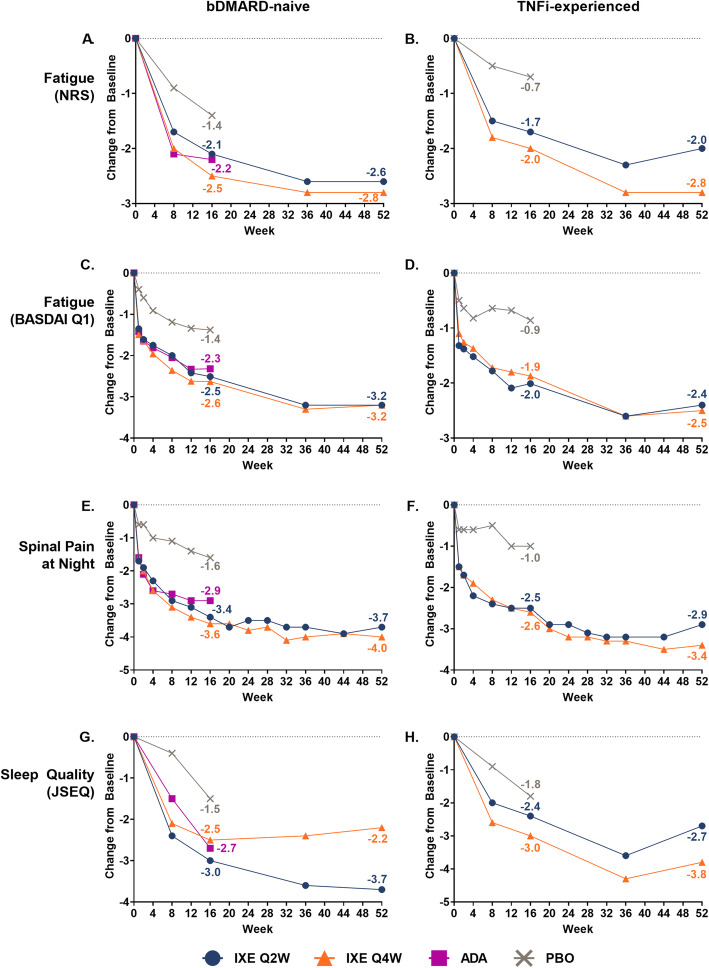
Fig. 2.
Changes from baseline in other PROs through 52 weeks of treatment. LSM (weeks 1–16) and mean (weeks 20–52) changes from baseline in other PROs for bDMARD-naïve (COAST-V) and TNFi-experienced (COAST-W) patients. The 16-week data have been published previously (10). At week 16, patients who originally received ADA or PBO were rerandomised 1:1 to ixekizumab Q2W or Q4W, while patients originally randomised to receive ixekizumab Q2W or Q4W continued those treatments. ADA = adalimumab; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; bDMARD = biologic disease-modifying antirheumatic drugs; IXE = ixekizumab; JSEQ = Jenkins Sleep Evaluation Questionnaire; LSM = least squares mean; NRS = numeric rating scale; PBO = placebo; PRO = patient-reported outcome; Q = question; Q2W = every 2 weeks; Q4W = every 4 weeks; TNFi = tumor necrosis factor inhibitor