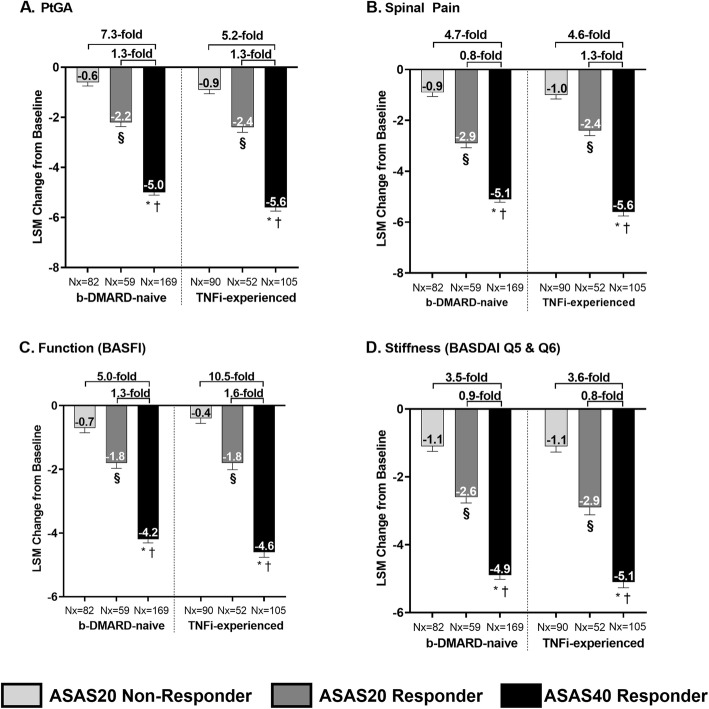
Fig. 3.
Association between ASAS response and improvements in ASAS PROs for ixekizumab-treated patients after 52 weeks. Q2W and Q4W ixekizumab-treated patients. § p < 0.001, achieved ASAS20 but not ASAS40 vs. ASAS20 not achieved; * p < 0.0001, ASAS40 achieved vs. ASAS20 not achieved; † p < 0.0001, ASAS40 achieved vs. achieved ASAS20 but not ASAS40. Results were compared using ANCOVA. Values are LSM improvements from baseline (SE). mBOCF was used for imputation of missing data. Fold difference = (ASAS40 responder/ASAS20 non-responder) -1, or (ASAS40 responder/ASAS20 but not ASAS40 responder) -1. ANCOVA = analysis of covariance; ASAS = Assessment of Spondyloarthritis International Society; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; BASFI = Bath Ankylosing Spondylitis Functional Index; bDMARD = biologic disease-modifying antirheumatic drugs; LSM = least squares mean; mBOCF = modified baseline observation carried forward; Nx = number of observations; PROs = patient-reported outcomes; PtGA = patient global disease activity; Q = question; Q2W = every 2 weeks; Q4W = every 4 weeks; SE = standard error; TNFi = tumor necrosis factor inhibitor