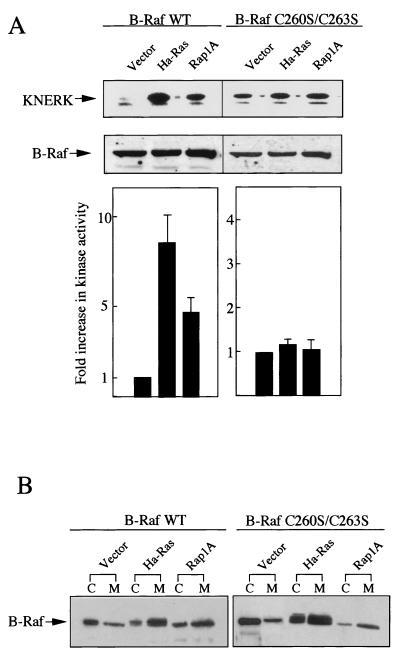
FIG. 2.
Ras- and Rap1A-dependent activation and membrane translocation of B-Raf and B-Raf(C260S/C263S). (A) pH8-FLAG-B-Raf (wild type [WT]) or pH8-FLAG-B-Raf(C260S/C263S) (0.5 μg of each) was cotransfected with either pEF-BOS-Ha-RasVal-12, pSRα-Rap1AVal-12, or pEF-BOS (3 μg of each) into COS7 cells. FLAG–B-Raf proteins were immunoprecipitated from the total cellular extract and examined for induction of phosphorylation of GST-KNERK in the presence of GST-MEK as described in Materials and Methods. The upper panel shows autoradiograms of phosphorylated GST-KNERK. The intensity of the KNERK bands was quantified with a BAS2000 bioimaging analyzer (lower panel) and expressed as fold increase with respect to the cells cotransfected with pEF-BOS. Immunoblot detection of B-Raf in the extracts is shown in the middle panel. The data shown are the means of three independent experiments. Standard deviations are indicated as error bars. (B) The transfected cells were homogenized and separated into cytosol (C) and membrane (M) fractions. B-Raf proteins present in the two fractions were detected by Western immunoblotting with anti-B-Raf antibody.