Abstract
A 47-year-old woman presented with a headache to the acute medical unit, 10 days after receiving AstraZeneca vaccination for COVID-19. Brain imaging was normal, but her blood tests showed a remarkably low platelet count, mildly deranged liver function tests and a high D-dimer. Further within her hospital admission, she developed right-sided abdominal pain and chest pain, and subsequent cross-sectional imaging confirmed a small segmental pulmonary embolism, and an acute portal vein thrombosis extending to the splenic and superior mesenteric veins. On the basis of her investigations, she was diagnosed as a case of vaccine-induced thrombotic thrombocytopenia and was treated with intravenous immunoglobulins. In a time where there is a strategic goal to vaccinate the global population from COVID-19 to inhibit the spread of infection and reduce hospitalisation, this particular clinical scenario emphasises the need of all clinicians to remain vigilant for rare complications of the COVID-19 vaccination.
Keywords: COVID-19, portal vein, immunological products and vaccines, pulmonary embolism, vaccination/immunisation
Background
The WHO declared the novel corona virus (COVID-19) outbreak as a global pandemic on 11th March 2020, following the first identified case in Wuhan, China in late December 2019.1 2
Vaccines offer a glimmer of hope in the fight against COVID-19, with many types of vaccines becoming increasingly available for use. One such type, the Oxford–AstraZeneca chimpanzee adenovirus-vectored vaccine ChAdOx1 nCoV-19 (AZD1222) provides particular hope for equitable access for low-income and middle-income countries. This is in comparison to the high cost of mRNA-focused vaccines that require storage in ultra-low temperature freezers and ultimately impractical for its use in many countries.3
The AstraZeneca vaccine was approved by the Medicine and Healthcare products Regulatory Agency (MHRA) on 30th December 20204 and was licensed for use in European countries by the European Medical Agency on 29th January 2021.5
In late February 2021, cases of unusual thrombotic events in combination with thrombocytopenia were emerging, in patients following vaccination with the AstraZeneca vaccine. As a result, many European countries initially suspended the use of the AstraZeneca vaccine while further analysis of the safety of the vaccine was conducted.6–8
We present, below, a case of an AstraZeneca-induced vaccine-induced thrombotic thrombocytopenia (VITT) involving two separate systemic circulations, resulting in direct hospitalisation. We highlight our radiological findings and the immunological-based management of her thrombotic disease.
Case presentation
A 47-year-old woman with a comorbidity only of the occasional migraine was invited to receive her first dose of the AstraZeneca vaccine at the end of March 2021. She received the vaccine into the deltoid muscle of her right arm.
Post vaccination, she reported no symptoms up until day 5, when she then developed right-sided periorbital pain, which progressed, gradually, to a generalised headache with mild photophobia, neck stiffness and lower back pain. There was no evidence of a neurological deficit, fevers or a new rash suggestive of meningitis. Sumatriptan did not alleviate her symptoms. Following a review by an out-of-hours general practitioner almost 10 days after her vaccination, she was referred to the acute medical team.
Further clinical history highlighted no personal or family history of thromboembolic disease. There was no history of miscarriages. The patient had a negative pregnancy urine test, and was not on any regular medications, in particular to contraceptive medications. There was no history of recent travel. Finally, she did not smoke tobacco or recreational drugs.
Investigations
Her blood tests demonstrated the following: (1) severe acute thrombocytopenia, with a platelet count of 13 × 109/L; (2) elevated D-dimer of >5000 ng/mL and (3) mildly deranged liver function tests with alanine transaminase of 57 U/L and alkaline phosphatase of 138 U/L. She had normal coagulation profile (international normalised ratio (INR) of 1 and an activated partial thromboplastin time (APTT) of 34.2). Her haemoglobin, white blood cell count, renal function tests and albumin were within normal parameters. Her reverse-transcription PCR testing via nasopharyngeal swab returned negative for COVID-19.
Considering her headache, a CT head was requested and showed no intracranial pathology. Further cross-sectional imaging via a MRI-venogram (figure 1) confirmed no evidence of a cerebral or dural thrombosis. Fundoscopy examination was normal. A lumbar puncture was muted, but not performed, considering her profound thrombocytopaenia.
Figure 1.
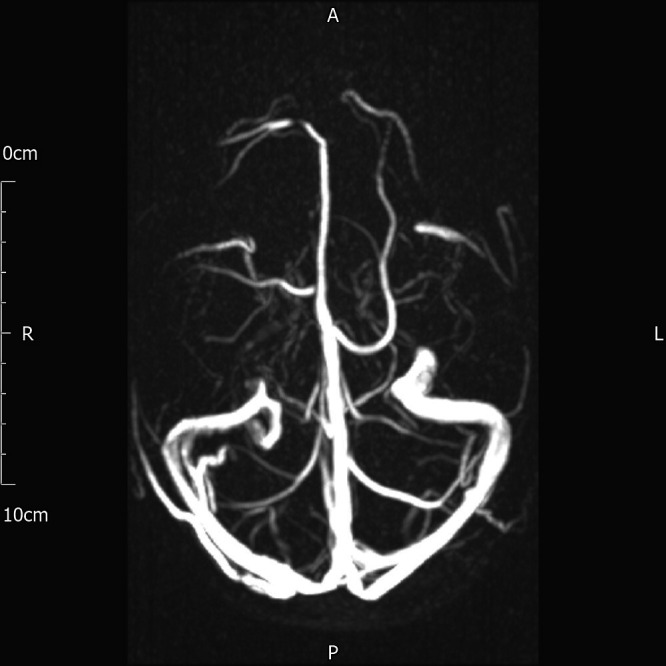
MRI-venogram: highlighted clear patency of cerebral vessels. A, anterior; P, posterior.
Considering her haemostasis parameters, a discussion with our haematology team ensued and she was quickly diagnosed as having a vaccine-associated thrombocytopenia. A PF4 antibody assay (ELISA) heparin-induced thrombocytopenia (HIT) assay was requested, and this returned as positive.
On day 5 of her admission, she developed acute right hypochondrial abdominal pain and lower chest pain. This resulted in an urgent triple phase scan of her liver, in addition to a CT pulmonary angiogram (CTPA).
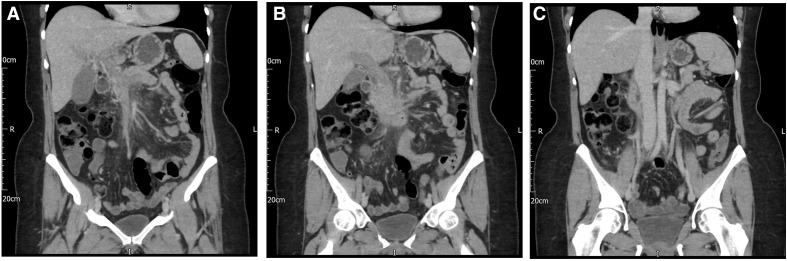
Subsequent cross-sectional imaging highlighted a small segmental right-sided pulmonary embolism (PE) (figure 2), and a completely occluded, dilated portal vein (figures 3A and 4A) with acute thrombosis extending into the superior mesenteric vein (figure 4B) and splenic vein (figures 3 and 4B), and the development of peri-portal collaterals. There was no evidence of underlying cirrhosis, with a preserved liver volume. There were no arterial enhancing liver lesions to suggest a primary liver tumour. There was no evidence of small or large bowel ischemia. Her final diagnosis was VITT.
Figure 2.

CTPA axial image showing a filling-defect in the right lower lobe pulmonary artery consistent with a pulmonary embolism.
Figure 3.
CT triple phase liver axial images showing (A) portal venous phase: distended and non-opacified portal vein (portal vein thrombosis) and (B) splenic vein thrombosis.
Figure 4.
CT triple phase liver: coronal images showing (A) dilated portal vein with complete thrombosis in the main branch, (B) splenic vein thrombosis and (C) normal appearing IVC and normal patency of the hepatic veins IVC, inferior vena cava.
Treatment
Considering her immunological-driven thrombotic disease, she was commenced on intravenous immunoglobulins (IVIGs) at a dose of 0.5 g/kg, over 2 days (days 3 and 5 of admission), and she received further IVIG on days 6 and 8 of admission.
Her platelet count improved rapidly, with a concomitant fall in her D-dimer levels. Symptomatically, she also improved in terms of her headache and abdominal pain. She was later commenced on Fondaparinox (day 7 of admission) and then switched to Apixaban (day 9 of admission) for further management of her thromboembolic disease. She was discharged after 12 days of admission.
Investigations (bloods tests), treatment and response to treatment are summarised in table 1.
Table 1.
Blood tests, dates of administration of IVIG and anticoagulation and summarises the response of treatment
| Day of admission | IVIG | Anticoagulation | Platelets (x 109/L) | D-dimer | ALT | ALP |
| Day 1 | 13 | >5000 | 57 | 138 | ||
| Day 2 | 16 | 105 | ||||
| Day 3 | 0.5 g/kg | 19 | 115 | |||
| Day 4 | >5000 | 100 | 154 | |||
| Day 5 | 0.5 g/kg | 19 | >5000 | 88 | ||
| Day 6 | 0.5 g/kg | 26 | 59 | 167 | ||
| Day 7 | 0.5 g/kg | Fondaparinox | 77 | >5000 | 46 | 188 |
| Day 8 | Fondaparinox | 132 | 3375 | 38 | 219 | |
| Day 9 | Apixaban | 190 | 2150 | 29 | 198 | |
| Day 10 | Apixaban | 263 | 317 | |||
| Day 11 | Apixaban | 282 | 2423 | 27 | 419 | |
| Day 12 | Apixaban | 1621 | 380 |
ALP, alkaline phosphatase; ALT, alanine transaminase; IVIG, intravenous immunoglobulin.
Outcome and follow-up
The patient completed an outpatient upper gastrointestinal endoscopy, which excluded oesophageal or gastric varices. She will remain under the dual care of hepatology and haematology teams.
Anticoagulation will be continued for a minimum of 3 months, with repeat cross-sectional imaging of her chest and abdomen scheduled in 3 months’ time.
The full thrombotic screen that was sent additionally (including anticardiolipin, lupus anticoagulant antibodies and thrombophilia screen), prior to IVIG treatment and use of anticoagulation, has come back as negative.
HIT antibodies came back as positive with percentage activation=31.57% (cut-off for positive reactions >8%).
Discussion
We describe a rare case of an immune-mediated severe thrombocytopenia and thrombosis involving the splanchnic and pulmonary vessels after the AstraZeneca vaccine (ChAdOx1 nCoV-19).
This case highlights the importance of recognition and management of rare complications in patients receiving the new COVID-19 vaccinations.
There are no previous case reports of COVID-19 vaccine-related splanchnic vein thrombosis within the UK; however, similar cases are reported in other parts of the world. Schultz et al9 reported case series of five patients in Norway with VITT after first dose of ChAdOx1 nCoV-19 adenoviral vector vaccine (AstraZeneca). These patients presented with venous thrombosis of unusual sites and concomitant thrombocytopenia within 7–10 days after receiving the first dose of the AstraZeneca vaccination. All patients had positive antibodies to platelet factor 4 (PF4)–polyanion complexes with no previous exposure to heparin. Four of five patients had severe cerebral venous thrombosis with intracranial haemorrhage, and the outcome was fatal in three of them. One of the patient presented with thrombosis of splanchnic circulation involving portal vein, splenic vein, azygos vein and hemiazygos vein. These five cases occurred in a population of >130 000 vaccinated persons.
Another such case was reported in Austria, whereby a 51-year-old woman developed PE and thrombus formation in the left internal iliac and common iliac veins, with extension into the inferior vena cava, after 11 days of receiving the AstraZeneca vaccination. This was associated with thrombocytopenia. HIT antibodies were not done in her case but the clinical picture fits with VITT.10
VITT following vaccination against SARS-CoV-2 with the AstraZeneca vaccine is an immune-mediated condition, that clinically resembles a severe HIT, but without the prior use of heparin.8 11
The hallmark of VITT is the presence of antibodies directed against PF4/heparin complexes. By their Fc domains, these immune complexes bind to FcγRIIA on the surface of platelets and thus cross-link these receptors and induce platelet activation.7
Cases emerging have shown that patients are presenting 5–28 days after vaccination and are characterised by severe thrombocytopenia, raised D-dimer levels, occasional low fibrinogen levels and progressive thrombosis at unusual sites. There is also evidence of the presence of antibodies to PF4, which can only be identified by ELISA HIT assay.
Current guidance provided by the Expert Haematology Panel (EHP; affiliated with the British Society of Haematology), focused on the management of COVID-19 VITT cases through past experience of managing similar (but not AstraZeneca-related) vaccine-associated conditions and the theoretical risks and benefits of interventions. IVIG is recommended to be commenced on an urgent basis at a dose of 1 g/kg (divided into 2 days if needed), irrespective of the degree of thrombocytopenia, and reviewing the clinical course closely. Further IVIG may be required balancing bleeding and thrombotic risk, with ongoing discussion with the haematologist. Platelet transfusion should be avoided. Once platelet counts improve >30×109/L, anticoagulation with non-heparin-based therapies such as direct-acting anticoagulants, argatroban, fondaparinux or danaparoid should be commenced. Steroids can be considered, in particular if there is a delay in giving IVIG.8 Plasma exchange may also be considered.
Anticoagulation should be continued for at least 3 months. All cases require formal reporting to the EHP and Public Health England and a yellow card needs to be filled for the Medicines and Healthcare products Regulatory Agency (MHRA).11
As discussed above, VITT involves development of antibodies against PF4/heparin complexes. When these immune complexes bind to FcγRIIA on the surface of platelets, platelet FcγRIIA stimulation leads to downstream activation of Bruton tyrosine kinase (Btk) as a decisive signalling pathway for subsequent steps of platelet activation. IVIG, which is a current approved treatment, inhibit platelet activation by shielding from FcγRIIA.
Philipp von Hundelshausen et al7 proposed in their recent article that inhibitors of Btk, which are used for B-cell malignancies, may become another therapeutic option for VITT, as they are expected to target multiple pathways downstream of FcγRIIA-mediated Btk activation, including effective inhibition of platelet aggregation, dense granule secretion, P-selectin expression and platelet-neutrophil aggregate formation stimulated by FcγRIIA cross-linking. Moreover, C-type lectin-like receptor CLEC-2-mediated and GPIb-mediated platelet activation; the interactions and activation of monocytes; and the release of neutrophil extracellular traps, as encountered in HIT, could all be attenuated by Btk inhibitors (Btki). In short, several activation mechanisms at the centre of VITT pathophysiology inhibited the Btki. Therefore, Btki might be regarded as a safe alternate for off-label use in selected cases of VITT.
Patient’s perspective.
The patient herself expressed a wish to provide a statement for this report.
I was not expecting that I will get such severe side effects from COVID-19 vaccination. I heard about the side effects but I thought it is very rare and would not happen to me as I am healthy. It has made me very scared for myself, and also for my family. The scariest part was that I did not get many symptoms except for the headache and I kept on thinking for first 5 days that I should not go to hospital, as it was not severe and seem insignificant. I would like people to know that one has to be vigilant after vaccination.
Learning points.
For general and acute physicians, the most important take home message is simply awareness of the condition. Mild-to-moderate constitutional symptoms such as fever, lethargy, myalgia and headache are common for up to 48 hours post vaccine. New or intense headache, symptoms of raised intracranial pressure (such as nausea, vomiting, visual changes or reduced consciousness), breathlessness, chest pain, abdominal pain, back pain, or leg pain, petechiae, prolonged bleeding or easy bruising all need to be investigated.
A strong recommendation is made to involve the haematology team early, to help guide management of suspected vaccine-induced thrombotic thrombocytopenia. Intravenous immunoglobulin is the first-line treatment and further doses may be needed if the platelet count continues to fall or D-dimer rise. Close follow-up will also be required with these patients.
Always report any cases identified using the yellow card system online.
Finally, if VITT is diagnosed or suspected, patients should not receive a second dose of the AstraZeneca vaccine.
Acknowledgments
We thank Dr Murty Vusirikala, Consultant Radiologist, Bedford Hospital, for providing the radiological images.
As Bedford Hospital and Luton and Dunstable Hospital have merged together to become one trust, we have used the Luton Hospital Fellowship code.
Footnotes
Contributors: The case was diagnosed and managed by gastroenterology and acute medial team, with JP and HA being the consultants and HA and FF being the registrars for the respective teams. HAs: she designed and wrote first draft of the manuscript including summary, introduction, case and discussion. She also complied the table of results and treatment and edited the manuscript as per her supervisor (JP) guidance and added images. Moreover, she spoke to the patient and took her personal statement and got her consent. She is also responsible for submitting the report to the BMJ. JP pointed out the case to be reported and supervised each step. He did critical revision of the manuscript, edited and approved the final version. He also provided the images by liaising with the radiologist. FF: he contributed to manuscript writing. HAl: he supervised and contributed to editing the manuscript and approved the final approval. All four authors approved the final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Cucinotta D, Vanelli M. Who Declares COVID-19 a pandemic. Acta Biomed 2020;91:157–60. 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He F, Deng Y, Li W. Coronavirus disease 2019: what we know? J Med Virol 2020;92:719–25. 10.1002/jmv.25766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021;397:72–4. 10.1016/S0140-6736(20)32623-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uk Government . Regulatory approval of COVID-19 vaccine AstraZeneca. Available: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca [Accessed 11 May 2021].
- 5.European Medicines Agency . Ema recommends COVID-19 vaccine AstraZeneca for authorisation in the EU. Available: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-astrazeneca-authorisation-eu [Accessed 11 May 2021].
- 6.Oldenburg J, Klamroth R, Langer F. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie 2021. [DOI] [PubMed] [Google Scholar]
- 7.von Hundelshausen P, Lorenz R, Siess W, et al. Vaccine-Induced immune thrombotic thrombocytopenia (VITT): targeting pathomechanisms with Bruton tyrosine kinase inhibitors. Thromb Haemost 2021. 10.1055/a-1481-3039. [Epub ahead of print: 13 Apr 2021]. [DOI] [PubMed] [Google Scholar]
- 8.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med Overseas Ed 2021;384:2124–30. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muster V, Gary T, Raggam RB, et al. Pulmonary embolism and thrombocytopenia following ChAdOx1 vaccination. Lancet 2021;397:1842. 10.1016/S0140-6736(21)00871-0 [DOI] [PubMed] [Google Scholar]
- 11.British Society for Haematology . Guidance produced from the expert haematology panel (Ehp) focussed on Covid-19 vaccine induced thrombosis and thrombocytopenia (VITT). Available: https://b-s-h.org.uk/media/19530/guidance-version-13-on-mngmt-of-thrombosis-with-thrombocytopenia-occurring-after-c-19-vaccine_20210407.pdf [Accessed 11 May 2021].