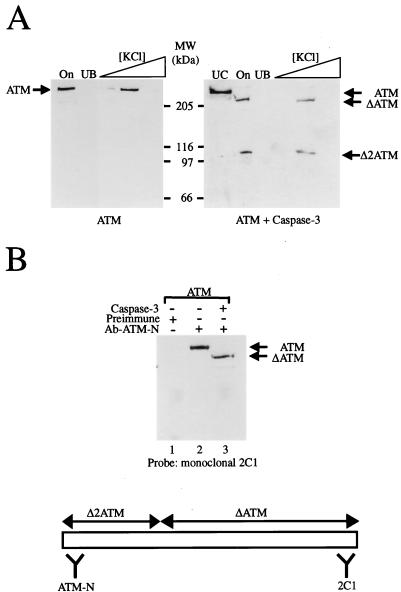
FIG. 7.
Proteolytically cleaved ATM fragments retain an ability to bind DNA and bind to each other. (A) Untreated (left-hand panel) or caspase 3-treated (right-hand panel) ATM was bound to DNA-coated iron-oxide particles, and then these were washed in 100 mM KCl to remove unbound material before elution of ATM protein with 150, 250, and then 500 mM concentrations of KCl. On, input material; UB, unbound material; UC, uncleaved ATM control; MW, molecular size markers. Samples were subjected to Western blot analysis with a mixture of anti-ATM antisera ATM-B and ATM-N. (B) Untreated or caspase 3-treated ATM pool was immunoprecipitated with either preimmune or ATM-N antiserum, as indicated. Following capture on protein A-Sepharose and extensive washing with TBS containing 0.1% NP-40, samples were processed by Western blotting with monoclonal antibody 2C1 that recognizes the C terminus of the ATM protein. Lane 1, ATM plus preimmune serum; lane 2, ATM plus antiserum ATM-N; lane 3, ATM plus caspase 3 plus antiserum ATM-N.