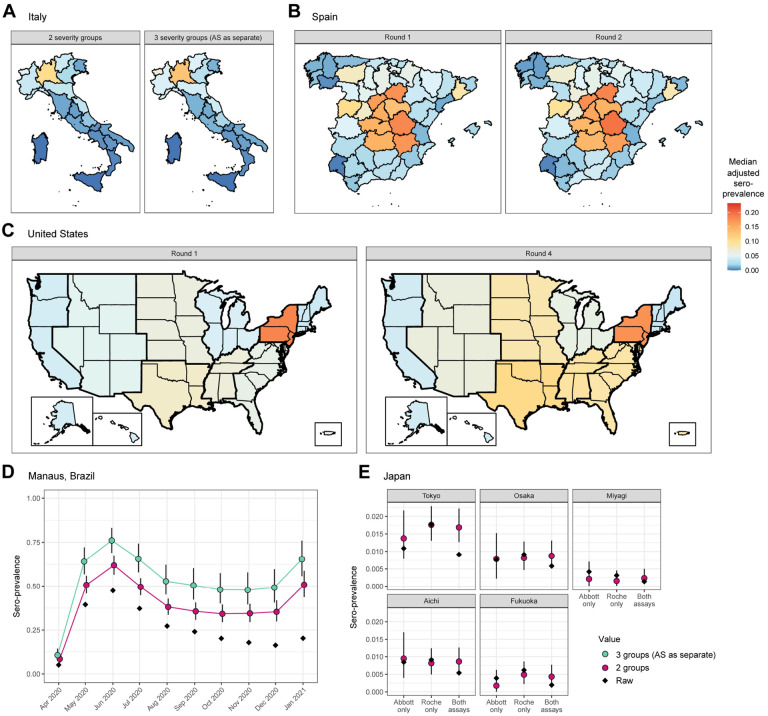
Figure 4: Adjusted seroprevalence estimates by survey.
Panels A, B, and C all share the same color scale. (A) Estimated seroprevalence by region in Italy, using the antibody kinetics model classifying disease severity into (left) 2 groups: non-hospitalized and hospitalized, and (right) 3 groups: asymptomatic (AS), symptomatic and non-hospitalized, and hospitalized. The former is considered as the primary scenario for this analysis, and the subsequent panels are derived under that scenario unless otherwise described. (B) Estimated seroprevalence by province in Spain for (left) Round 1 and (right) Round 2 of the serosurvey. (C) Estimated seroprevalence by census division in the United States for (left) Round 1 and (right) Round 4 of the serosurvey, incorporating weighting by the population demographics (age and sex) of each census division, as well as age-specific probabilities of hospitalization. (D) Estimated seroprevalence by month in Manaus, Brazil, incorporating weighting by age and sex, as well as age-specific probabilities of hospitalization and of experiencing symptoms, restricted to between the ages of 15 and 70 years. The raw seropositive proportion is in black; results from the antibody kinetics model classifying disease severity into 2 groups are in pink, and 3 groups are in green. (E) Estimated seroprevalence for the 5 prefectures in Japan, considering the raw results from the (left) Abbott assay only, (center) Roche assay only, and (right) both assays. The raw seropositive proportion is in black and the estimated seroprevalence is in pink. When considering the results from both assays, the raw seropositive proportion is the proportion of samples that tested positive on both.