Abstract
Kappa opioid receptor (KOR) agonists produce robust analgesia with minimal abuse liability and are considered promising pharmacological agents to manage chronic pain and itch. The KOR system is also notable for robust differences between the sexes, with females exhibiting lower analgesic response than males. Sexually dimorphic traits can be due to either the influence of gonadal hormones during development or adulthood, or due to the complement of genes expressed on the X or Y chromosome. Previous studies examining sex differences in KOR antinociception have relied on surgical or pharmacological manipulation of the gonads to determine whether sex hormones influence KOR function. While there are conflicting reports whether gonadal hormones influence KOR function, no study has examined these effects in context with sex chromosomes. Here, we use two genetic mouse models, the Four Core Genotypes (FCG) and XY*, to isolate the chromosomal and hormonal contributions to sex differences in KOR analgesia. Mice were treated with systemic KOR agonist (U50,488H) and thermal analgesia measured in the tail withdrawal assay. We found that KOR anti-nociception was influenced predominantly by the number of the X chromosomes. These data suggest that the dose and/or parental imprint on X gene(s) contribute significantly to the sexually dimorphism in KOR analgesia.
Keywords: sex difference, sex chromosomes, sex hormones, kappa opioid receptor, analgesia
Introduction
Activation of the kappa opioid receptor (KOR) produces analgesia without euphoria and is emerging as a target for chronic pain and itch without abuse potential. The KOR system is also notable for a significant sex difference, with females less responsive to KOR agonists than males (Barrett et al., 2002; Craft & Bernal, 2001; S. Stevens Negus et al., 2002; Stoffel et al., 2005). This is important because females are more likely to suffer from chronic pain and require pain treatments (Sorge & Totsch, 2017). Understanding the biological basis for the sex difference in KOR agonist efficacy is necessary to promote analgesic effects of agonists for treatment of pain in females.
The biological origins of the sex differences in KOR analgesia remain elusive. Sexually dimorphic traits can be due to either the influence of gonadal hormones during development or adulthood (Arnold, 2017). In addition, the complement of genes expressed on the X or Y chromosome can drive, or contribute to, sexually dimorphic phenotypes (Arnold, 2017). The influence of gonadal hormones on KOR function is complex. For example, gonadectomy has been shown to decrease KOR antinociception in both males and females and fluctuating female gonadal hormones influence KOR spinal antinociception (Abraham et al., 2018; S. S. Negus & Mello, 2002; Stoffel et al., 2005). However, additional reports have indicated that KOR activation and agonist-induced dysphoria does not vary with estrous cycle nor following gonadectomy of either ovaries or testes (Conway et al., 2019; Harris & Drake, 2001; Russell et al., 2014).
The studies above have relied on surgical or pharmacological manipulation of the gonads, which is unable to isolate underlying sex chromosome influences. In fact, it is a challenge to isolate these chromosomal effects, given that sex chromosomes are always expressed in the presence of their respective gonads. To overcome this limitation, we use two genetic approaches to manipulate the expression of sex chromosomes independent of gonadal hormones. In the “Four Core Genotypes” (FCG), the testes determining gene, Sry, is removed from the Y chromosome and reinserted as a transgene on chromosome 3 (Burgoyne & Arnold, 2016). This produces 4 genotypes comprised of males and females each with either XX or XY sex chromosomes. Because gonadal sex is independent of sex chromosomes, conclusions can be drawn about the role of gonadal hormones and sex chromosomes. If a sex difference is found to vary based on sex chromosomes, the FCG model cannot differentiate whether this difference is due to genes on the X or Y chromosome. The latter question can be answered by using the XY* model in which male mice have a variant Y chromosome (Y*) that recombines abnormally with the X chromosome during meiosis. Mating an XY* mouse with a WT XX female produces 4 genotypes in which the dose of X and Y genes varies independently (i.e.: XX, XO, XY, and XXY). This model provides information on whether the sex difference varies with the number of X or Y chromosomes (Burgoyne & Arnold, 2016).
In this study, we use the FCG and XY* genetic lines to determine the biological basis for the sex differences in KOR anti-nociception. By manipulating sex chromosomes and gonadal hormones independently, we identify the role of sex chromosomes, gonadal hormones, and the interaction between the two in mediating KOR analgesia.
Methods
Animals
All animal care and experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and use of Laboratory animals and were approved by the University of California Los Angeles Institutional Animal Care and Use Committee and the University of Alberta Health Sciences Animal Care and Use Committee.
Wild type male and female C57BL/6J (8 weeks, total 44 mice equal males and females) mice were ordered from Jackson Laboratories. Four Core Genotype (FCG, total 53 mice 6-8 per genotype) mice on a C57BL/6J (B6) background (B6.Cg-Tg(Sry)2Ei Srydl1Rlb/ArnoJ, Jackson Laboratories stock 10905; backcross generation greater than 23) bred at UCLA. In this model, the testis-determining gene Sry is deleted from the Y chromosome and inserted as a transgene on chromosome 3 (Itoh et al., 2015). FCG mice comprise four genotypes: XX and XY females (without Sry, with ovaries) and XX and XY males (with Sry and testes). Because gonadal sex is independent of sex chromosome complement, conclusions can be drawn about the role of gonadal hormones and sex chromosome complement (and interactions between the two) in the observed sex difference. In this study, “male” refers to a mouse with Sry (with testes) and “female” refers to a mouse without Sry (with ovaries) and is independent of sex chromosome complement (XX or XY). In some animals, gonadectomy was performed at 75 days of age. Following isoflurane anesthesia (1.5-3%), gonads were exposed and excised. Animals were allowed to recover in their home cages for at least 2 weeks prior to testing.
The XY* model (total 35 mice, 6-11 per genotype), backcrossed to C57BL6/J, was used to isolate which sex chromosome was responsible for the sex differences in KOR anti-nociception (Burgoyne et al., 1998). XX gonadal females were bred with XY* gonadal males. This produces four genotypes XY*X, XX, XY* and XXY* that are similar to the genotypes XO, XX, XY, and XXY, respectively (Burgoyne & Arnold, 2016; Burgoyne et al., 1998; Chen et al., 2008; Eicher et al., 1991). While XX and XO mice have ovaries (female), XXY and XY mice have testes (male). The genotypes allow measurement of the effects of one vs. two X chromosomes (XO vs. XX; XY vs. XXY) and effects of one vs. no Y chromosome (XY vs. XO; XXY vs. XX).
In both models, mice were separated by gonadal sex and housed in groups of four. All experiments were performed with mice between 8-12 weeks of age (25-35 g). All animals were housed in ventilated plastic cages in groups of four with standard bedding, maintained on normal 12 h light/dark cycle (temperature at 22-C and 60% humidity) with lights on a 07:00h, and allowed free access to standard rodent chow (Teklad, Harland, Indianapolis, IN, USA) and water.
Genotyping
For the FCG, ear punches were collected to isolate DNA. Extracted DNA was amplified using polymerase chain reaction (PCR) using a commercial PCR mix (Promega) and specific primers for the Sry gene, the Y chromosome and internal control (Burgoyne & Arnold, 2016). For the XY* mice, the number of X chromosomes and the presence of the Y chromosome were determined by interphase fluorescent in situ hybridization using gene markers specific for the X and Y chromosome (Kreatech KI-30505 kit, Leica Biosystems). Some genotypes were confirmed by anatomical assessment, including anogenital distance and testis size.
Drugs
U50,488H hydrochloride (U50) was purchased from R&D Systems (Minneapolis, MN, USA). Ethylketocyclazocine (EKC) was purchased from Sterling Drug Inc (Rensselaer, NY, USA). All drugs were dissolved in saline (0.9% sodium chloride) and given by sub-cutaneous (s.c.). injection (0.22-0.32mL per injection, titrated to body weight).
Tail withdrawal assay
Antinociception was measured by the tail withdrawal assay. Briefly, animals were gently retrained in a soft plastic conical sleeve and 2.5cm of the tail was immersed in 49°C water. The time to tail withdrawal was measured. After three baseline measurements, mice received an injection of U50 (10 mg/kg, s.c.) or EKC (1 mg/kg, s.c.). The latency to tail withdrawal was measured every 10 minutes for 30 minutes. A cut-off of 15 seconds was imposed to avoid tissue damage. For the dose response curve, mice were injected with escalating doses of U50 (1-30 mg/kg, s.c.) or EKC (0.1-10 mg/kg, s.c.), and tail withdrawal latencies were measured after 20 minutes, immediately prior to the next injection. Withdrawal latencies were converted to percent maximum potential effect (%MPE) calculated as [test latency – baseline latency]/[cut-off latency-baseline latency]*100.
Data Analysis
Results are shown as means +/− SEM. Data was tested for normality (Shapiro-Wilk Normality Test) and means compared with one-, two- or three-way ANOVA, as required. Differences were considered significant when p<0.05.
Results
Sex-Dependent KOR Anti-nociception
Female C57BL/6J mice exhibited significantly lower baseline tail withdrawal thresholds (Fig. 1a; t=2.38 (18), p = 0.03, n=10) and significantly lower U50,488H (10 mg/kg, s.c.) anti-nociception (Fig. 1b; Fsex(1,10)=8.7, p = 0.01, n=10). This sex difference remained when data were normalized to baselines and expressed as the percent maximum potential effect (%MPE, Fsex(1, 18)=8.12, p=0.01). Following cumulative dose response testing, females exhibited a reduction in maximal effect of U50,488H (Fig 1c; Fsex(1,30)=10.87, p=0.003, n=6; Emax females: 4.5 versus males: 11.13, p=0.044). To determine whether this sex difference was unique to U50,488H, another KOR agonist (EKC; 1 mg/kg, s.c.) was used under the same conditions. This resulted in a similar sex difference, with females exhibiting a significantly lower antinociceptive response than males (Fig. 1d, Fsex(1,10)=1.68, p=0.22, n=6, Fig 1e: t(10)2.58, p=0.02, n=6, Fig 1e; Fsex(1,32)=7.44, p=0.01, n=6).
Figure 1: KOR anti-nociception is lower in female mice.
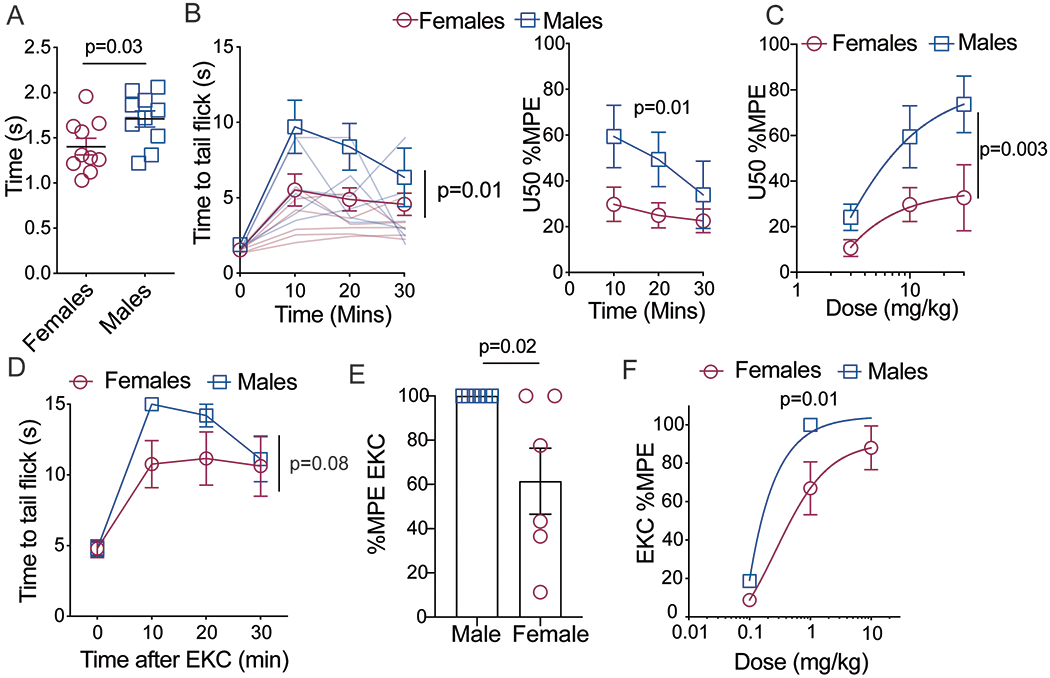
A) Baseline tail withdrawal thresholds are significantly higher in male C57BL/6J mice, n=10. B) U50 anti-nociception (10mg/kg) is significantly lower in female mice. Left graph is raw data indicating tail withdrawal thresholds from 49oC water. Right graph is transformed data (Percent maximum potential effect, %MPE), n=10. C) U50 dose response curve indicates rightward shift in females, n=6 D) Sex differences exist with another KOR agonist, ethylketocyclazocine (EKC, 1mg/kg, s.c.). Tail withdrawal thresholds from 49oC water were measured before and after injection with EKC E) Transformed data (%MPE) of tail withdrawal thresholds from animals treated with EKC (1mg/kg, s.c.) 10 minutes after drug injection, n=6. F) Females exhibit a rightward shift in the EKC dose response curve, n=6. Data expressed as means +/− SEM.
Hormonal versus Chromosomal Contribution to Sex Difference
To determine whether the observed sex difference in U50,488H anti-nociception is due to gonadal hormones or sex chromosome genes, the sex difference in KOR function was further investigated using FCG mice. Baseline tail withdrawal thresholds were significantly higher in XY animals than XX, regardless whether they were male (testes, +Sry) or females (ovaries, -Sry) (Fig. 2a; FChromosome(1,22)=24.50, p=<0.0001; FHormone(1,22)=0.33, p=0.56; FInteraction(1,22)=7.56, p=0.01, n=6-8). Following U50,488H administration (10 mg/kg, s.c.), XY animals exhibited greater tail flick antinociception when compared to XX animals (Fig. 2b; FChromosome(1,22)=11.84, p=0.002; FHormone(1,22)=0.20, p=0.20, Finteraction(1,22)=0.65, p=0.43, n=6-8). This effect remained when data were transformed to %MPE (FChromosome(1,22)=7.32, p=0.01, FHormone(1,22)=1.23, p=0.28, Finteraction(1,22)=0.006, p=0.93, n=6-8) Although there was no main effect of gonad type, the interaction between sex chromosome complement and gonad type on baseline tail withdrawal thresholds (Figure 2a) suggests that ovarian hormones may augment the sex chromosome effect, or testicular hormones may blunt it, or both. Nevertheless, removing the gonads from the FCG mice did not reduce the overarching sex difference seen in the tail flick latencies (Fig. 2c; FGonadectomy (1,43)=0.87, p=0.35, FChromosome(1,43)=13.5, p<0.0001; FHormone(1,43)=0.92, p=0.34, no significant interactions detected, n=5-8).
Figure 2: KOR antinociception varies with sex chromosome, not gonadal hormones.
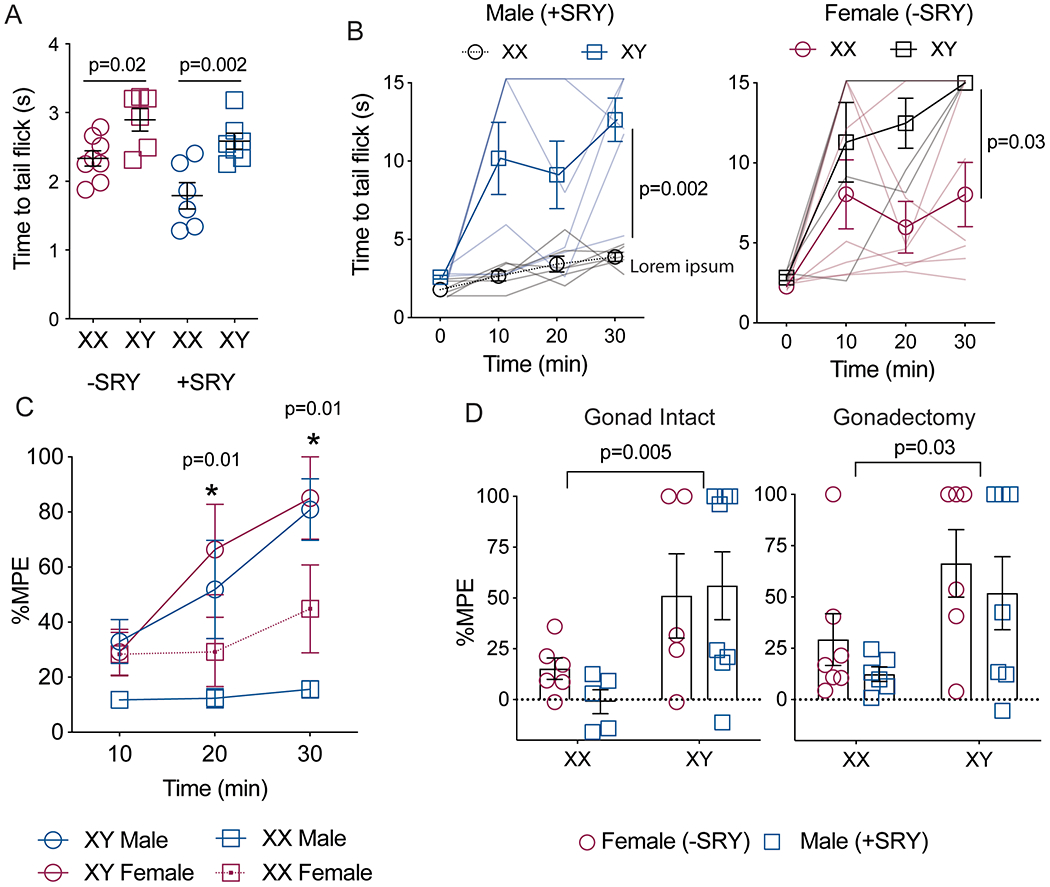
A) Baseline tail withdrawal thresholds vary by sex chromosome and gonadal hormone, with animals with a Y chromosome displaying higher baseline pain withdrawal thresholds than animals with two X chromosomes, n=6-8. B) U50 anti-nociception (10mg/kg, s.c.) is significantly higher in XY than XX mice, regardless of type of gonad. Data were compared with three-way ANOVA with time, sex chromosome, and gonadal hormone as independent factors. Males and females are plotted separately for clarity, n=6-8 C) Tail withdrawal thresholds were transformed to percent maximum potential effect (%MPE), n=6-8. D) Removal of gonads does not affect U50 sex differences in tail withdrawal, n=5-8. Data presented as mean =/− SEM.
X versus Y Chromosome Contribution to Sex Difference
To determine whether this sex difference is due to genes on the X chromosome or the Y chromosome, the XY* model was used. Baseline tail flick latencies were not significantly different based on number of X chromosomes or the presence or absence of a Y chromosome (Fig. 3a; FY(1,34)=0.25, p=0.62; FX(1,34)=1.03, p=0.32, Finteraction(1,34)=3.40, p=0.07, 6-11). There was no significant difference in U50,488H anti-nociception (10mg/kg, s.c.) between mice with or without a Y chromosome (1X females versus 1X males; 2X females versus 2X males), but there was a significantly lower U50,488 anti-nociception in mice with 2X chromosomes compared to mice with 1 X chromosome (1X females versus 2X females; Fig. 3b; FX dose(1,47)=6.23, p=0.02, FY dose (1,47)=0.57, p=0.40, FInteraction(1,47)=0.5, p=0.38, n=6-11). This effect remained when data were transformed to %MPE (FX dose(1,31)=4.43, p=0.044, FY dose(1,31)=0.61, p=0.44, Finteraction(1,31)=1.13, p=0.30, n=6-11).
Figure 3: KOR antinociception varies with the X chromosome, not Y chromosome.
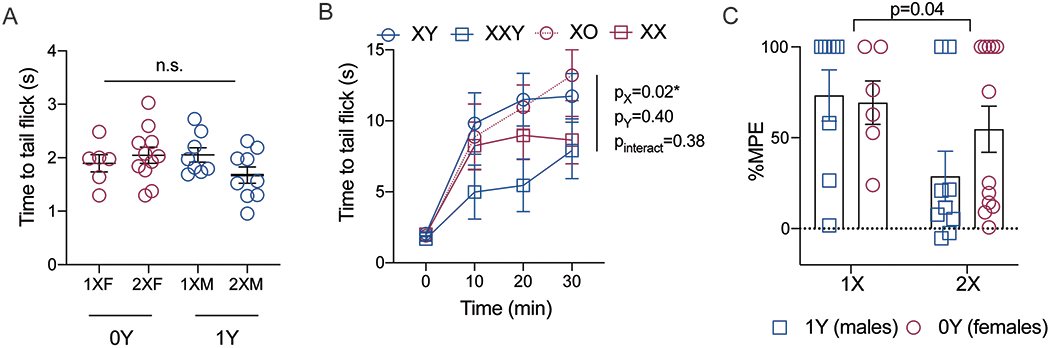
A) In the XY*model, baseline tail withdrawal latencies were not significantly between any of the genotypes, n=6-11. B) U50 antinociception (10mg/kg, s.c.) tended to be lower in animals with two X chromosomes compared to 1 X chromosome. The presence or absence of the Y chromosome (SRY) did not affect withdrawal thresholds, n=6-11. C) Tail withdrawal thresholds 20 minutes following U50 administration were transformed to percent maximum effect (%MPE), n=6-11. Data presented as mean +/− SEM. Data were compared with 2 or 3-way ANOVA, with time, X chromosome, and Y chromosome as independent factors.
Discussion
Using genetic manipulation of the X and Y chromosomes, we assessed the contribution of sex chromosomes and gonadal hormones on KOR analgesia. We found that in FCG mice, XX animals exhibited significantly less KOR analgesia than XY animals. This chromosomal effect occurred in mice with testes or ovaries. The difference was replicated in the XY* model, which revealed that the XX-XY difference was caused by differences in the number of X chromosomes, not by the presence of absence of the Y chromosome.
Sex hormones did not significantly influence KOR anti-nociception in either gonad-intact or gonadectomized animals (Fig 2). This suggests that the activational and organizational effects of gonadal hormones play a minor role in this observed sex difference. While this seemingly contradicts published literature describing activational effects of female gonadal hormones on KOR analgesia (Abraham et al., 2018; Clemente-Napimoga et al., 2009), we did observe a significant interaction between gonadal hormones and sex chromosomes in mediating baseline withdrawal thresholds, which indicate gonadal hormones may augment or inhibit the sex chromosome effect. Moreover, given that previous reports indicate that the magnitude of KOR sex difference depends on species, strain, and pain modality tested (Barrett et al., 2002; Craft & Bernal, 2001; Liu et al., 2013), the contribution of gonadal hormones to KOR sex differences may well depend upon such factors. Finally, it should be emphasized that this study does not directly assess whether flucutations in gonadal hormones contribute to observed sex differences. We did not assess female reproductive cycle nor did we measure gonadal hormone levels in individual animals. Therefore, it is possible gonadal hormones influence KOR analgesia within the context of the sex chromosome complement, and more research is warranted to investigate these processes. KOR agonists also effectively activate the hypothalamic-pituitary-adrenal axis, and we have shown previously that a significant portion of KOR analgesia is driven by stress-induced analgesia (Taylor et al., 2015). Future studies are needed to determine whether the sex chromosome complement also contributes to sex differences in KOR activity in stress pathways. It is unlikely that differences in KOR analgesia between males and females are due to pharmacokinetic differences, given that U50,488H metabolism and brain concentrations have been shown to be the same between the sexes (Laman-Maharg 2018, Russell et al).
Our results are the first to indicate that sex chromosomes contribute an important portion of the sex differences in KOR analgesia. We found that genes located on the X chromosome contribute to lowered KOR anti-nociception in females (Fig 3). Our results also indicate a significant interaction between X chromosomes genes and gonadal hormones (Fig 2a), which suggests this X chromosome effect is more robust in gonadal males over females. While this study does not identify the specific genes responsible, there are some intriguing possibilities. The mouse X chromosome contains over a thousand genes. However, due to the process of X inactivation, only a minority of genes on the X chromosome are bi-allelically expressed in females (X escapees). In the rodent, 13 X chromosome genes are potential escapees (Berletch et al., 2011). Of these 13, 6 express mRNA at higher levels in the XX than XY brain (Xu et al., 2002). Most of these genes are involved in transcriptional regulation, and are widely expressed across tissues, and thus may have wide ranging influence on many other genes and proteins. Particularly interesting are two X escapee genes, Kdm6a and Kdm5c, which are expressed higher in XX than XY cells from the brain, immune system, adipose tissues, liver, and heart (Chen et al., 2012; Itoh et al., 2019; Li et al., 2014; Xu et al., 2008). These genes are histone demethylases, and thus have broad epigenetic effects across the genome. Recently sex differences in expression of Kdm6a have been implicated in causing sex differences in mouse models of bladder cancer (Kaneko & Li, 2018) and autoimmune diseases (Itoh et al., 2019). These and other X escapees are top candidate for regulation of KOR analgesia (Arnold, 2017; Golden et al., 2019).
One intriguing possibility is that X chromosome genes control the expression or function of KOR. Indeed, sex differences in KOR agonist binding and activity have been noted. For example, PET imaging using radiolabeled KOR ligand ([11C]LY2795050) indicate men had higher KOR availability than women in multiple brain regions, including cerebellum, frontal cortex, and parietal cortex (Vijay et al., 2016). In animal studies, autoradiography with tritiated KOR agonist U69,593, showed greater binding in males throughout the brain, including somatosensory and insular cortices (Wang et al., 2011). KOR have also been shown to be differentially regulated in males and females in chronic pain (Liu et al., 2019). Finally, immunocytochemical analysis of downstream signaling events (p44/p42 MAPK) indicate reduced KOR receptor activation throughout the brain, but particularly in the amygdala (Rasakham et al., 2012). This is of interest given the importance of the amygdala in modulating pain and stress responses (Kang-Park et al., 2015; Nation et al., 2018).
In conclusion, our study indicates that genes on the X chromosome contribute to observed sex difference in KOR analgesia between males and females. These results indicate fundamental differences KOR signaling, driven in part, by genes located on the X chromosome.
Supplementary Material
Significance Statement:
Kappa opioid receptor (KOR) agonists are known to have a notable sex difference; however, the biological basis for this sex difference remained elusive. Previous work relying on surgical or chemical manipulation of gonadal hormones have produced conflicting results on the relative contribution of gonadal hormones to the KOR sex difference. Here, we were able to show that sex chromosomes (and particularly the X chromosome) contributes significantly to the KOR sex difference. This discovery carries meaningful clinical implications given KOR agonists are currently being developed as novel analgesics, and women are significantly more likely to require pain treatments in their lifetime.
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Council of Canada (NSERC Discovery Grant, AMWT), the Shirley and Stefan Hatos Foundation for Neuropharmacology (AMWT, CJE), NIH Grant 1R01HD076125 (APA) and DA005010 (CJE).
Grant Information: Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (AMWT), NIH NICHD 1R01HD076125 (APA), Shirley and Stefan Hatos Foundation for Neuropharmacology (AMWT, CJE)
Footnotes
Conflict of Interest Statement: None of the authors have any conflict of interest to declare.
Data Accessibility Statement: The authors confirm that the data supporting the findings of this study are available within the article.
References
- Abraham AD, Schattauer SS, Reichard KL, Cohen JH, Fontaine HM, Song AJ, … Chavkin C (2018). Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion. J Neurosci, 38(37), 8031–8043. doi: 10.1523/JNEUROSCI.0653-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP (2017). A general theory of sexual differentiation. J Neurosci Res, 95(1–2), 291–300. doi: 10.1002/jnr.23884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Smith ES, & Picker MJ (2002). Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur J Pharmacol., 452(2), 163–173. [DOI] [PubMed] [Google Scholar]
- Berletch JB, Yang F, Xu J, Carrel L, & Disteche CM (2011). Genes that escape from X inactivation. Hum Genet, 130(2), 237–245. doi: 10.1007/s00439-011-1011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, & Arnold AP (2016). A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol Sex Differ, 7, 68. doi: 10.1186/s13293-016-0115-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, & Ashworth A (1998). The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet, 80, 37–40. [DOI] [PubMed] [Google Scholar]
- Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, & Reue K (2012). The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet, 8(5), e1002709. doi: 10.1371/journal.pgen.1002709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Watkins R, Delot E, Reliene R, Schiestl RH, Burgoyne PS, & Arnold AP (2008). Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev Neurobiol, 68(2), 265–273. doi: 10.1002/dneu.20581 [DOI] [PubMed] [Google Scholar]
- Clemente-Napimoga JT, Pellegrini-da-Silva A, Ferreira VH, Napimoga MH, Parada CA, & Tambeli CH (2009). Gonadal hormones decrease temporomandibular joint kappa-mediated antinociception through a down-regulation in the expression of kappa opioid receptors in the trigeminal ganglia. Eur J Pharmacol, 617(1–3), 41–47. doi: 10.1016/j.ejphar.2009.06.036 [DOI] [PubMed] [Google Scholar]
- Conway SM, Puttick D, Russell S, Potter D, Roitman MF, & Chartoff EH (2019). Females are less sensitive than males to the motivational- and dopamine-suppressing effects of kappa opioid receptor activation. Neuropharmacology, 146, 231–241. doi: 10.1016/j.neuropharm.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, & Bernal SA (2001). Sex differences in opioid antinociception: kappa and ‘mixed action’ agonists. Drug Alcohol Depend, 63, 215–228. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, King TR, … Washburn LL (1991). The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet, 57(4), 221–230. doi: 10.1159/000133152 [DOI] [PubMed] [Google Scholar]
- Golden LC, Itoh Y, Itoh N, Iyengar S, Coit P, Salama Y, … Voskuhl RR (2019). Parent-of-origin differences in DNA methylation of X chromosome genes in T lymphocytes. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1910072116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, & Drake CT (2001). Kappa opioid receptor density is consistent along the rostrocaudal axis of the female rat spinal cord. Brain Res, 905(1–2), 236–239. doi: 10.1016/s0006-8993(01)02515-x [DOI] [PubMed] [Google Scholar]
- Itoh Y, Golden LC, Itoh N, Matsukawa MA, Ren E, Tse V, … Voskuhl RR, (2019). The X-linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J Clin Invest, 130, 3852–3863. doi: 10.1172/JCI126250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Mackie R, Kampf K, Domadia S, Brown JD, O’Neill R, & Arnold AP (2015). Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes, 8, 69. doi: 10.1186/s13104-015-0986-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, & Li X (2018). X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci Adv, 4(6), eaar5598. doi: 10.1126/sciadv.aar5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, & Moore SD (2015). Interaction of CRF and kappa opioid systems on GABAergic neurotransmission in the mouse central amygdala. J Pharmacol Exp Ther, 355(2), 206–211. doi: 10.1124/jpet.115.225870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen X, McClusky R, Ruiz-Sundstrom M, Itoh Y, Umar S, … Eghbali M (2014). The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovasc Res, 102(3), 375–384. doi: 10.1093/cvr/cvu064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NJ, Schnell S, Wessendorf MW, & Gintzler AR (2013). Sex, pain, and opioids: interdependent influences of sex and pain modality on dynorphin-mediated antinociception in rats. J Pharmacol Exp Ther, 344(2), 522–530. doi: 10.1124/jpet.112.199851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, … Cahill CM (2019). Kappa Opioid Receptors Drive a Tonic Aversive Component of Chronic Pain. J Neurosci, 39(21), 4162–4178. doi: 10.1523/JNEUROSCI.0274-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation KM, De Felice M, Hernandez PI, Dodick DW, Neugebauer V, Navratilova E, & Porreca F (2018). Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain, 159(5), 919–928. doi: 10.1097/j.pain.0000000000001167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, & Mello NK (2002). Effects of gonadal steroid hormone treatments on opioid antinociception in ovariectomized rhesus monkeys. Psychopharmacology (Berl), 159(3), 275–283. doi: 10.1007/s002130100912 [DOI] [PubMed] [Google Scholar]
- Negus SS, Zuzga DS, & Mello NK (2002). Sex differences in opioid antinociception in rhesus monkeys: Antagonism of fentanyl and U50,488 by quadazocine. The Journal of Pain, 3(3), 218–226. doi: 10.1054/jpai.2002.124734 [DOI] [PubMed] [Google Scholar]
- Rasakham K, McGillivray KL, & Liu-Chen LY (2012). Sex differences in U50,488H-induced phosphorylation of p44/42 mitogen-activated protein kinase in the guinea pig brain. Neuroscience, 223, 447–456. doi: 10.1016/j.neuroscience.2012.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, & Chartoff EH (2014). Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry, 76(3), 213–222. doi: 10.1016/j.biopsych.2013.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, & Totsch SK (2017). Sex Differences in Pain. J Neurosci Res, 95(6), 1271–1281. doi: 10.1002/jnr.23841 [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Folk JE, Rice KC, & Craft RM (2005). Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain, 6(4), 261–274. doi: 10.1016/j.jpain.2004.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AMW, Roberts KW, Pradhan AA, Akbari HA, Walwyn W, Lutfy K, … Evans CJ (2015). Anti-nociception mediated by a kappa opioid receptor agonist is blocked by a delta receptor agonist. Br J Pharmacol, 172(2), 691–703. doi: 10.1111/bph.12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay A, Wang S, Worhunsky P, Zheng M-Q, Nabulsi N, Ropchan J, … Morris ED (2016). PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo. Am J Nucl Med Mol Imaging, 6(4), 205–214. [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, & Liu-Chen LY (2011). Sex difference in kappa-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5’-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J Pharmacol Exp Ther, 339(2), 438–450. doi: 10.1124/jpet.111.183905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Burgoyne PS, & Arnold AP (2002). Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet, 11(12), 1409–1419. doi: 10.1093/hmg/11.12.1409 [DOI] [PubMed] [Google Scholar]
- Xu J, Deng X, & Disteche CM (2008). Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS One, 3(7), e2553. doi: 10.1371/journal.pone.0002553 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


