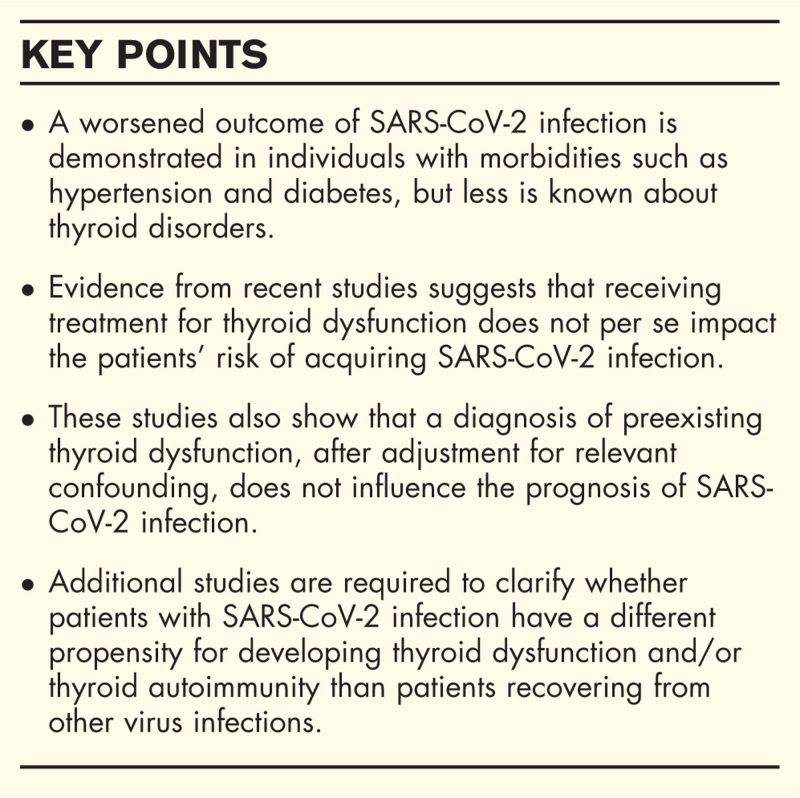
Purpose of review
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is associated with excess morbidity and mortality in patients with hypertension and diabetes but little is known about thyroid diseases. Thus, our goal was to review the literature with respect to: (i) Are patients with underlying hypo- or hyperthyroidism at increased risk of contracting SARS-CoV-2 infection? (ii) do underlying hypo- and hyperthyroidism impact the prognosis of SARS-CoV-2 infection? (iii) does SARS-CoV-2 infection cause de novo thyroid dysfunction?
Recent findings
Patients with hypo- or hyperthyroidism do not have an increased risk of contracting SARS-CoV-2, and a diagnosis of hypo- or hyperthyroidism is not associated with a worsened prognosis of SARS-CoV-2 infection. SARS-CoV-2 infection has been associated with subsequent thyrotoxicosis, euthyroid sick syndrome, subacute thyroiditis, and autoimmune thyroid disease.
Summary
These findings suggest that receiving treatment for thyroid dysfunction does not per se impact the patients’ risk of acquiring SARS-CoV-2 infection, or the management of those who already contracted it. Additional studies with larger numbers of patients and long-term follow-up are required in order to clarify whether patients with SARS-CoV-2 infection are more or less prone to develop thyroid dysfunction and/or thyroid autoimmunity than patients recovering from other virus infections.
Keywords: coronavirus disease 2019, hyperthyroidism, hypothyroidism, severe acute respiratory syndrome coronavirus-2, thyroid dysfunction
INTRODUCTION
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection [1] has spread dramatically worldwide and is associated with excess morbidity and mortality. By end of April 2021, the virus had affected more than 140 million people and claimed more than 3 million lives. Identification of factors contributing to the risk of contracting SARS-CoV-2, and the subsequent prognosis in those affected, is important in order to optimize the reallocation of hospital resources, and guide public health recommendations and interventions. Hospital-based case series [2–4] as well as population-based cohort studies [5,6] have found old age, male gender, and presence of a wide range of comorbidities, especially hypertension and diabetes, the main risk factors for severe disease and death. Whether underlying hypo- or hyperthyroidism influence the risk and/or course of SARS-CoV-2 infection is less clear. Both the American Thyroid Association [https://www.thyroid.org/covid-19/statement-covid-19] and the European Thyroid Association [https://www.eurothyroid.com/files/download/ETA-PHB.pdf] have issued consensus statements to patients receiving treatment for thyroid dysfunction. Although these recommendations were not anchored in strong clinical evidence-based data, they advise patients to continue their prescribed medications of Levothyroxine (L-T4) and antithyroid drugs (ATD) for hypo- and hyperthyroidism, respectively. As these conditions are common [7], any increased risk of contracting SARS-CoV-2 and/or a worse prognosis of SARS-CoV-2 infection will have major public health impact.
This review interprets results from relevant studies in order to answer three clinical questions: (i) Are patients with underlying hypo- or hyperthyroidism at increased risk of contracting SARS-CoV-2 infection? (ii) do underlying hypo- and hyperthyroidism impact the prognosis of SARS-CoV-2 infection? (iii) does SARS-CoV-2 infection cause de novo thyroid dysfunction?
Box 1.
no caption available
UNDERLYING THYROID DYSFUNCTION AND RISK AND COURSE OF SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2 INFECTION
Theoretically, patients with hypo- or hyperthyroidism may have an increased risk of contracting and/or developing a severe course of COVID-19. First, as SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) as a receptor for host cell entry [8], thyroid dysfunction may influence the risk and course of COVID-19 because the activity of ACE2 is influenced by serum levels of thyroid hormones [9,10]. Second, patients with hypo- or hyperthyroidism have an increased burden of somatic [11,12], especially cardiovascular [13,14], and psychiatric [15,16] co-morbidity, which is also reported in patients with severe and fatal COVID-19 [2,3,5,6]. Third, in most patients the etiology of hypo- and hyperthyroidism is of autoimmune origin (95% and 50% for hypo- and hyperthyroidism, respectively) [17,18]. Although patients with autoimmune disorders do not appear to be more likely to contract COVID-19 [19,20▪▪], they may have severe complications if their immune system is suppressed by their medication [19,21], e.g., risk of agranulocytosis in patients treated with ATD [22]. It remains debated whether the aforementioned conditions translate into increased risk of acquiring or a more severe prognosis of SARS-CoV-2 infection in patients with hypo- or hyperthyroidism.
THYROID DYSFUNCTION AND RISK OF CONTRACTING SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2 INFECTION
During the first 6 months of the COVID-19 pandemic, there was virtually no data on the risk of COVID-19 in patients with underlying thyroid dysfunction. By the second half of 2020, large-scale population-based epidemiological data have quantified the risk of contracting SARS-CoV-2 infection among patients diagnosed with Graves’ disease (GD) [20▪▪] and in patients treated for hypo- or hyperthyroidism [23▪]. Table 1 outlines the results of these studies. In order to investigate the prevalence of SARS-CoV-2 infection in patients with various autoimmune diseases, Attauabi et al.[20▪▪] analyzed, all individuals tested for SARS-CoV-2 between January 28 and June 2, 2020 in two Danish regions (Capital and Zealand). During the study period, 14 (0.2%) of 8.476 SARS-CoV-2 positive patients and 419 (0.2%) of 223.125 SARS-CoV-2 negative persons were classified as having GD. Prevalence of those testing positive for SARS-CoV-2 did not differ between those with GD and the background population (3.2% vs 3.7%, P = 0.65). Using data from the Danish COVID-19 cohort [24], Brix et al.[23▪] performed a nationwide population-based study to explore the risk of contracting SARS-CoV-2 in patients receiving medical treatment for hypo- or hyperthyroidism. The study included all individuals testing positive for SARS-CoV-2 (n = 28,078) in Denmark between February 27 and September 30, 2020. Each of the 28,078 positive individuals were matched with up to 10 SARS-CoV-2 negative individuals on age, sex, and week of testing. 809 (2.9%) of the 28,078 positive patients and 7,994 (2.9%) of the 280,007 matched SARS-CoV-2 negative controls were treated for hypothyroidism (P = 0.81), whereas 91 (0.3%) and 936 (0.3%) of SARS-CoV-2 positive and negative persons, respectively, were treated for hyperthyroidism (P = 0.78). Patients treated for hypo- or hyperthyroidism did not have an increased risk of contracting SARS-CoV-2 infection [ORhypo = 1.03 (95% CI 0.95–1.11) and ORhyper = 1.03 (0.82–1.28)]. These findings accord with those observed in other autoimmune diseases, such as inflammatory bowel disease, psoriasis, multiple sclerosis, rheumatoid arthritis, and coeliac disease [20▪▪,25,26].
Table 1.
Number and prevalence of patients and type of thyroid dysfunction in SARS-CoV-2 positive and negative populations
| SARS-CoV-2 positive population | SARS-CoV-2 negative population | |||||||
| Study | Phenotype | Thyroid disease | No thyroid disease | Prevalence of thyroid disease | Thyroid disease | No thyroid disease | Prevalence of thyroid disease | P value |
| Attauabi et al.[20▪▪] | Graves’ disease | 14 | 8,476 | 0.2% | 419 | 223,125 | 0.2% | 0.58 |
| Brix et al.[23▪] | Users of ATDa | 91 | 28,078 | 0.3% | 936 | 280,007 | 0.3% | 0.78 |
| Users of thyroxine | 809 | 28,078 | 2.9% | 7.994 | 280,007 | 2.9% | 0.81 | |
ATD, antithyroid drugs (methimazole, carbimazole, and propylthiouracil).
THYROID DYSFUNCTION AND PROGNOSIS OF SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2 INFECTION
Findings from a meta-analysis of eight retrospective hospital-based observational studies have suggested that patients with thyroid disease are at increased risk of worsened outcomes of SARS-CoV-2 infection [27]. However, six studies included < 10 patients with a thyroid condition [28–33], three studies did not differentiate between hypo- and hyperthyroid individuals [32–34], and one study did not include patients with SARS-CoV-2 infection [29], hampering any guidance of patients and physicians.
Subsequently, two controlled studies have evaluated the course of SARS-CoV-2 infection in patients with various thyroid conditions, such as hypothyroidism [35▪], and patients treated for either hypo- or hyperthyroidism [23▪]. Table 2 summarizes the findings of these studies. Using data from the New York Healthcare system and including both hospitalized and ambulatory patients, van Gerwen et al.[35▪] examined whether the course of 3703 patients with SARS-CoV-2 was influenced by preexisting hypothyroidism. They identified 251 patients with a diagnosis of hypothyroidism. After adjusting for age, sex, race, BMI, smoking status and a number of comorbidities, using a propensity score method, the risk of hospitalization [0.76 (0.58–1.00)], use of mechanical ventilation [0.85 (0.58–1.25)], and death [1.04 (0.71–1.52)] did not differ between patients with and without a diagnosis of hypothyroidism [35▪]. In a nationwide cohort of 16.502 SARS-CoV-2 positive patients diagnosed between February 27 and August 31, 2020, Brix et al.[23▪] ascertained 572 (3.5%) and 75 (0.5%) SARS-CoV-2 positive patients who were current users of L-T4 and ATD, respectively. In order to evaluate the prognosis, patients with and without use of L-T4/ATD were followed prospectively until 30 days after their positive SARS-CoV-2 test. To minimize bias and confounding the analyses were adjusted for age, sex and various comorbidities using propensity score weighting. Compared with nonusers, current users of L-T4 had an increased risk of being hospitalized [1.19 (1.02–1.40)] and undergoing dialysis [2.23 (1.06–4.69)]. However, when extending the follow-up to 60 days and taking different test strategies into account these associations attenuated, and the authors concluded that current use of L-T4 did not impact the prognosis of the SARS-CoV-2 infected. Likewise, current use of ATD was not associated with adverse outcomes of SARS-CoV-2 infection (Table 2).
Table 2.
Characteristics of and results from controlled studies evaluating course of SARS-CoV-2 infection in patients with preexisting thyroid disease
| Studies | ||
| Van Gerwen et al.[35▪] | Brix et al.[23▪] | |
| Country | USA | Denmark |
| Setting | New York City healthcare system | Nationwide |
| Time frame | March 1 to April 1, 2020 | February 27 to August 31, 2020 |
| Confounder control | Age, sex, race, BMI, smoking, and comorbidity | Age, sex, time of testing, and comorbidity |
| Thyroid conditions (n) | Hypothyroidism (251) | Use of levothyroxine (572) Use of antithyroid drugs (75) |
| Outcomes | Odds ratioa (95% CI) | Risk ratioa (95% CI) |
| Hospitalization | 0.76 (0.58–1.00) | 1.19 (1.02–1.40)b,c 1.15 (0.77–1.71)d |
| Death | 1.04 (0.71–1.52) | 0.87 (0.65–1.17)b 1.04 (0.62–1.73)d |
| Admission to intensive care unit | 1.34 (0.86–2.08)b 0.90 (0.23–3.60)d | |
| Mechanical ventilation | 0.85 (0.58–1.25) | 1.32 (0.79–2.22)b 1.35 (0.34–5.39)d |
| Dialysis | 2.23 (1.06–4.69)b,c 3.62 (0.86–15.14)d | |
Propensity score weighted.
Users of levothyroxine.
When using a 60 day follow-up these associations attenuated; RR = 1.31 (0.92–1.85) and 3.91 (0.93–16.37) for hospitalization and dialysis, respectively.
Users of antithyroid drugs.
CRITICAL APPRAISAL AND INTERIM CONCLUSIONS
Large-scale controlled studies show that patients with underlying thyroid dysfunction are no more prone to SARS-CoV-2 infection than the background population. These studies also suggest that preexisting thyroid dysfunction, when controlling for relevant confounding, does not influence the prognosis of SARS-CoV-2 infection. Although the data quality in these studies [20▪▪,23▪,35▪] is high, the results robust and pointing in the same direction, some limitations deserve attention. First, as patients with hypo- and hyperthyroidism, irrespective of cause, have an increased burden of SARS-CoV-2 related co-morbidities [11,12], many will be classified as vulnerable or high-risk patients and be advised to exhibit cautious behavior to limit risk of becoming infected. Thus, it cannot be ruled out that patients with underlying thyroid dysfunction may have been more observant than persons without thyroid disease, and as a consequence have limited their risk of contracting SARS-CoV-2 infection. Second, in the study by Attauabi et al.[20▪▪] GD patients were identified based on a record in The Danish National Patient Registry. This registry only covers patients diagnosed in the secondary healthcare system, questioning the generalizability of these findings to other ascertainment situations and populations. Moreover, the authors offer no information on the timeframe between the diagnosis of GD and the SARS-CoV-2 test, rendering it impossible to evaluate whether the patients had active disease or were in remission on the date of the SARS-CoV-2 test. Third, all patients in the study by Brix et al.[23▪], and most likely the majority of patients in the other controlled studies [20▪▪,35▪], received medical treatment for their underlying thyroid disease. Therefore, it is conceivable that the majority of patients were euthyroid, or that the severity of dysfunction at the time of the SARS-CoV-2 test was minor and without influence on the risk of contracting and/or worsening the course of SARS-CoV-2 infection. Fifth, in three studies [23▪,34,35▪] no information on the cause of hypo- or hyperthyroidism is provided, making it impossible to assess whether the risk and prognosis of SARS-CoV-2 infection differ between autoimmune and nonautoimmune thyroid dysfunction. Sixth, valid data on risk and course of SARS-CoV-2 in patients with thyroid dysfunction originate from relatively affluent countries such as Denmark [20▪▪,23▪] and the USA [35▪], both characterized by a well-financed healthcare system. It remains to be shown whether these findings hold when expanding investigations to include other settings.
CAN SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2 INFECTION CAUSE DE NOVO THYROID DYSFUNCTION?
Theoretically, SARS-CoV-2 infection could lead to subsequent thyroid dysfunction. First, viral infections, including influenza [36], human immunodeficiency virus [37], and other coronaviruses such as SARS-CoV [38], have been cited as environmental factors involved in subacute thyroiditis and autoimmune thyroid diseases [39–41]. Second, alterations in thyroid function tests, especially low triiodothyronine (T3) also known as Nonthyroidal illness syndrome (NTI), occur in many with a significant acute disease [42]. Third, ACE2 is present in the thyroid gland [43,44], making the thyroid susceptible to tissue injury as observed in other organs such as the lungs, gastrointestinal duct, and myocardium, which also harbor high levels of ACE2 [43]. Therefore, the observation of thyroid dysfunction in patients hospitalized due to SARS-CoV-2 infection [45▪,46,47▪,48▪,49–52], is of no surprise. The relevant clinical questions are, do variations in thyrotropin (TSH) and thyroid hormones occur more often in patients with SARS-CoV-2 infection compared with other acute conditions, i.e., sepsis and are the variations observed in levels of TSH and thyroid hormones specific for SARS-CoV-2 infection?
PREVALENCE, SEVERITY AND SPECIFICITY OF THYROID DYSFUNCTION DURING SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2 INFECTION
Thyrotoxicosis, hypothyroidism, and low serum TSH have been reported in up to 20%, 5%, and 39%, respectively, in patients hospitalized with SARS-CoV-2 infection [46,49,52]. However, these studies did not include a control population, hampering interpreting the SARS-CoV-2-specific pattern and potential clinical relevance of these findings. Three studies (summarized in Table 3) have compared the prevalence and severity of thyroid dysfunction in patients hospitalized with SARS-CoV-2 with a relevant control population [45▪,47▪,48▪].
Table 3.
Level of thyroid function tests and prevalence of thyroid dysfunction in hospitalized patients with and without SARS-CoV-2 infection
| Levels of various thyroid variables in SARS-CoV-2 positive vs SARS-CoV-2 negative | ||||||
| Study | Country | Control population | TSH | T-3 | T-4 | Prevalence of thyroid dysfunctiona in SARS-CoV-2 patients vs controls |
| Chen et al.[45▪] | China | 1) Healthy controls 2) Non-SARS-CoV-2 pneumonia | Lower Lower | Lower Lower | No difference No difference | Low TSH, 56% vs ?%, P < 0.01b |
| Muller et al.[47▪] | Italy | Non-COVID-19 patients treated at an ICUc | Lower | No difference | No difference | Thyrotoxicosis, 15% vs 1%, P < 0.01 Low TSH, 25% vs 8%, P < 0.01 Suppressed TSH, 9% vs 1%, P < 0.01 Hypothyroidism, 4% vs 9%, P = 0.51 |
| Khoo et al.[48▪] | England | Patients with clinical features of COVID-19 but with a negative SARS-CoV-2 test | Lower | No data | Lower | Hyperthyroidism, 0% vs 0%d Hypothyroidism, 1% vs 0%d Subclinical hyperthyroidism, 5%vs 7%d Subclinical hypothyroidism, 5% vs 6%d |
Thyroid dysfunction defined as per the original publications.
No data for the frequency of low TSH in the two control populations are reported. The p-value represents comparison between cases and the healthy controls. Results for comparison of cases and patients with non-SARS-CoV-2 pneumonia are not reported.
Intensive care unit.
The proportions of patients within each phenotype did not differ significantly between SARS-CoV-2 positive and negative.
In the retrospective study by Chen et al.[45▪], thyroid function parameters in 50 hospitalized patients with SARS-CoV-2 were compared with those of a healthy control group matched for age and sex, and a group of non-COVID-19 pneumonia patients. At admission (defined as within 3 days), 56% (28/50) of hospitalized SARS-CoV-2 positive patients without previous thyroid diseases, showed lower-than-normal values for TSH (<0.3 mIU/L). Although, actual numbers for the two control groups are not given, this finding was reported significant when compared with the healthy control group. Unfortunately, the authors do not report comparison of the frequency of low TSH between SARS-CoV-2 cases and the non-COVID-19 pneumonia patients. The SARS-CoV-2 infected had significantly lower levels of TSH and total T3 as compared both with the healthy subjects and those with non-COVID-19 pneumonia. Total T4 levels did not differ between the three groups (Table 3). The authors interpreted these findings as best explained by NTI.
A different thyroid pattern was described by Muller et al. in Italy [47▪] and Khoo et al. in England [48▪]. Muller et al.[47▪] investigated thyroid function variables in 85 consecutive SARS-CoV-2 patients admitted to an intensive care unit (ICU) from 3 March to 28 April 2020. Patients treated in the same unit during the equivalent timeframe in 2019 served as controls. Thyroid function was assessed within 2 days of hospital admittance and a significantly higher prevalence of thyrotoxicosis (15%), low (25%), or suppressed TSH (9%), compared to 1%, 8%, and 1%, respectively, in the control group was reported. There was no difference in the prevalence of hypothyroidism between the two groups (Table 3). Levels of TSH were significantly lower in SARS-CoV-2 patients than in controls, whereas free T3 and free T4 were similar, and interpreted as a combination of thyrotoxicosis, due to atypical thyroiditis, and NTI. Yet another picture is reported by Khoo et al.[48▪], comparing thyroid function variables (within 2 days after hospital admission) between 334 patients with SARS-CoV-2 infection and 122 patients with a clinical suspicion of COVID-19 but with a negative test. The prevalence of thyroid dysfunction was similar between patients with and without SARS-CoV-2 infection (Table 3). Levels of TSH and free T4 were significantly lower among patients with SARS-CoV-2 as compared to patients testing negative, despite having clinical symptoms. The authors concluded that there was no evidence for a SARS-CoV-2 related thyroiditis/thyrotoxicosis, and their findings most likely explained by NTI.
CRITICAL APPRAISAL AND INTERIM CONCLUSIONS
All controlled studies [45▪,47▪,48▪] investigating SARS-CoV-2 infection and subsequent thyroid function show that those infected have significantly lower TSH values than the controls. This consistency may suggest a specific role of SARS-CoV-2 infection on the thyroid or the pituitary. However, interpretation of serological data need be cautious as these cannot appoint SARS-CoV-2 as responsible. Additionally, although including relevant control populations, it is very likely that the SARS-CoV-2 patients had more grave disease and were treated differently (i.e. use of glucocorticoids) than the controls. When it comes to levels of T3 and T4, the picture is less clear. Importantly, the age and sex distribution, iodine status of the background population, the threshold for hospitalization, admission to ICU, and treatment (i.e. use of glucocorticoids, heparin, and dopamine), hampering head to head comparison of the studies. Finally, these studies [45▪,47▪,48▪] only cover hospitalized patients and do not allow speculation on whether nonhospitalized patients with SARS-CoV-2 infection have alterations in their thyroid function.
THYROID DYSFUNCTION AFTER SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS-2 INFECTION
Data on thyroid function following hospitalization for SARS-CoV-2 infection are limited [45▪,47▪,48▪]. In the Chinese study [45▪] all thyroid variables normalized following recovery. Likewise, Khoo et al.[48▪] showed that all 55 patients with data on thyroid function before admission, during hospitalization, and after a median follow-up of 79 days normalized their thyroid function parameters during recovery. Muller et al.[47▪] reevaluated thyroid function in eight SARS-CoV-2 patients who had thyroid dysfunction at hospital admission. After a mean follow-up of 55 days, two patients (25%) had hypothyroidism, whereas the remaining six patients (75%) had normal thyroid function and negative thyroid autoantibodies. When this is said, a growing number of case stories report the development of thyroid dysfunction classified as subacute thyroiditis [53–55], postpartum thyroiditis [56], and GD [57–59] following SARS-CoV-2 infection. Accepting the dogma that subacute thyroiditis and autoimmune thyroid diseases are related to prior viral infection [39,41], this is not surprising. However, lacking large-scale studies investigating the thyroid consequences following SARS-CoV-2 infection makes it impossible to evaluate whether these patients are more or less prone to develop thyroid dysfunction and/or thyroid autoimmunity than patients recovering from other virus infections.
CONCLUSION
Patients with hypo- or hyperthyroidism do not have an increased risk of contracting SARS-CoV-2, and when adjusted for comorbidity, a diagnosis of hypo- or hyperthyroidism is not associated with a worsened prognosis of SARS-CoV-2 infection. The clinical implications of and recommendations following these findings are that receiving treatment for thyroid dysfunction should not per se impact the patients’ risk of acquiring SARS-CoV-2 infection, or the management of those who already contracted it. Although this information is based on robust data, a number of issues remain to be clarified. Thus, whether etiology of the thyroid dysfunction [60], magnitude of thyroid dysfunction before/during infection, and cumulative period of thyroid dysfunction before infection, which clearly influence mortality [61,62] also influence the risk and prognosis of SARS-CoV-2 infection are unknown. In patients hospitalized due to SARS-CoV-2 infection, alterations in thyroid function parameters reflecting thyrotoxicosis and NTI have been observed. Development of subacute thyroiditis and autoimmune thyroid disease following SARS-CoV-2 infection have also been reported, but additional studies with larger numbers of patients and long-term follow-up are needed to substantiate these observations.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021; 19:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. The N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020; 323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang BY, Barnard LM, Emert JM, et al. Clinical characteristics of patients with coronavirus disease 2019 (COVID-19) receiving emergency medical services in King County, Washington. JAMA Netw Open 2020; 3:e2014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reilev M, Kristensen KB, Pottegård A, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol 2020; 49:1468–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 2020; 8:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol 2018; 14:301–316. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diniz GP, Senger N, Carneiro-Ramos MS, et al. Cardiac ACE2/angiotensin 1-7/Mas receptor axis is activated in thyroid hormone-induced cardiac hypertrophy. Ther Adv Cardiovasc Dis 2016; 10:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis PJ, Lin HY, Hercbergs A, et al. Coronaviruses and integrin αvβ3: does thyroid hormone modify the relationship? Endocr Res 2020; 45:210–215. [DOI] [PubMed] [Google Scholar]
- 11.Thvilum M, Brandt F, Almind D, et al. Type and extent of somatic morbidity before and after the diagnosis of hypothyroidism. a nationwide register study. PLoS One 2013; 8:e75789.doi: 10.1371/journal.pone.0075789. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandt F, Thvilum M, Almind D, et al. Morbidity before and after the diagnosis of hyperthyroidism: a nationwide register-based study. PLoS One 2013; 8:e66711.doi: 10.1371/journal.pone.0066711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lillevang-Johansen M, Abrahamsen B, Jorgensen HL, et al. Duration of over- and under-treatment of hypothyroidism is associated with increased cardiovascular risk. Eur J Endocrinol 2019; 180:407–416. [DOI] [PubMed] [Google Scholar]
- 14.Lillevang-Johansen M, Abrahamsen B, Jorgensen HL, et al. Duration of hyperthyroidism and lack of sufficient treatment are associated with increased cardiovascular risk. Thyroid 2019; 29:332–340. [DOI] [PubMed] [Google Scholar]
- 15.Brandt F, Thvilum M, Almind D, et al. Hyperthyroidism and psychiatric morbidity: evidence from a Danish nationwide register study. Eur J Endocrinol 2014; 170:341–348. [DOI] [PubMed] [Google Scholar]
- 16.Thvilum M, Brandt F, Almind D, et al. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid 2014; 24:802–808. [DOI] [PubMed] [Google Scholar]
- 17.Carle A, Laurberg P, Pedersen IB, et al. Epidemiology of subtypes of hypothyroidism in Denmark. EurJ Endocrinol 2006; 154:21–28. [DOI] [PubMed] [Google Scholar]
- 18.Carle A, Pedersen IB, Knudsen N, et al. Epidemiology of subtypes of hyperthyroidism in Denmark: a population-based study. EurJ Endocrinol 2011; 164:801–809. [DOI] [PubMed] [Google Scholar]
- 19.Emmi G, Bettiol A, Mattioli I, et al. SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun Rev 2020; 19:102575.doi: 10.1016/j.autrev.2020.102575. Epub 2020 May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Attauabi M, Poulsen A, Theede K, et al. Prevalence and outcomes of COVID-19 among patients with inflammatory bowel disease-A Danish Prospective Population-based Cohort Study. J Crohns Colitis 2021; 15:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]; By utelizing nonbiased data from a number of Danish health databases, this large scale population based study provide valid estimates for risk and prognosis of SARS-CoV-2 infection in patients with inflammatory bowel disease and various other autoimmune diseases, including Graves’ disease. Their results have important impact in guiding health recommendations and interventions in patients with autoimmune diseases during the COVID-19 pandemic.
- 21.Tan EH, Sena AG, Prats-Uribe A, et al. COVID-19 in patients with autoimmune diseases: characteristics and outcomes in a multinational network of cohorts across three countries. Rheumatology 2021; 0:1–14. doi:10.1093/rheumatology/keab250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith TJ, Hegedus L. Graves’ Disease. N Engl J Med 2016; 375:1552–1565. [DOI] [PubMed] [Google Scholar]
- 23▪.Brix TH, Hegedüs L, Hallas J, Lund LC. Risk and course of SARS-CoV-2 infection in patients treated for hypothyroidism and hyperthyroidism. Lancet Diabetes Endocrinol 2021; 9:197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]; By utilizing nonbiased data from a number of Danish health databases, this nationwide population based study provide valid estimates for risk and prognosis of SARS-CoV-2 infection in patients receiving treatment for underlying hypo- and hyperthyroidism. In this study, patients treated for hypo- or hyperthyroidism did not have an increased risk of contracting SARS-CoV-2, and treatment for hypo- or hyperthyroidism is not associated with a worse prognosis of SARS-CoV-2 infection. These results have important impact in guiding health recommendations and interventions in patients receiving treatment for thyroid dysfunction during the COVID-19 pandemic.
- 24.Pottegård A, Kristensen KB, Reilev M, et al. Existing data sources in clinical epidemiology: The Danish COVID-19 Cohort. Clin Epidemiol 2020; 12:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yiu ZZN, Harding G, Griffiths CEM, et al. Risk of COVID-19 infection in adult patients with atopic eczema and psoriasis: a single centre, cross-sectional study. Br J Dermatol 2021; doi: 10.1111/bjd.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarmiento-Monroy JC, Espinosa G, Londoño MC, et al. A multidisciplinary registry of patients with autoimmune and immune-mediated diseases with symptomatic COVID-19 from a single center. J Autoimmun 2021; 117:102580.doi: 10.1016/j.jaut.2020.102580. Epub 2020 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hariyanto TI, Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr 2020; 14:1429–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao M, Zhang D, Wang Y, et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. MedRxiv 2020; 2020.03.04.20030395. doi: 10.1101/2020.03.04.20030395. Preprint. [Google Scholar]
- 29.Liu J, Wu X, Lu F, et al. Low T3 syndrome is a strong predictor of poor outcomes in patients with community-acquired pneumonia. Sci Rep 2016; 6:22271.doi: 10.1038/srep22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shabrawishi M, Al-Gethamy MM, Naser AY, et al. Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia. PLoS One 2020; 15:e0237130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Liu Y, Liu L, et al. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at Hospital Admission in Shenzhen, China. J Infect Dis 2020; 221:1770–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan S, Song X, Lin F, et al. Clinical characteristics of coronavirus disease 2019 in Hainan, China. MedRxiv 2020; doi.org/10.1101/2020.03.19.20038539. [Google Scholar]
- 33.Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020; 75:1730–1741. [DOI] [PubMed] [Google Scholar]
- 34.Sisó-Almirall A, Kostov B, Mas-Heredia M, et al. Prognostic factors in Spanish COVID-19 patients: a case series from Barcelona. PLoS One 2020; 15:e0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪.van Gerwen M, Alsen M, Little C, et al. Outcomes of patients with hypothyroidism and COVID-19: a retrospective cohort study. Front Endocrinol 2020; 11:565.doi: 10.3389/fendo.2020.00565. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using data from the New York Healthcare system, this study examined whether the course of 3703 patients with SARS-CoV-2 was influenced by a preexisting diagnosis of hypothyroidism. After adjusting for age, sex, race, BMI, smoking status and relevant comorbidities, the risk of hospitalization, use of mechanical ventilation, and death did not differ between patients with and without a diagnosis of hypothyroidism. Their results have important impact in guiding recommendations and interventions in patients with hypothyroidism during the COVID-19 pandemic.
- 36.Dimos G, Pappas G, Akritidis N. Subacute thyroiditis in the course of novel H1N1 influenza infection. Endocrine 2010; 37:440–441. [DOI] [PubMed] [Google Scholar]
- 37.Bouillet B, Petit JM, Piroth L, et al. A case of subacute thyroiditis associated with primary HIV infection. Am J Med 2009; 122:e5–e6. [DOI] [PubMed] [Google Scholar]
- 38.Wei L, Sun S, Xu CH, et al. Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol 2007; 38:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J 2009; 6:5.doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speer G, Somogyi P. Thyroid complications of SARS and coronavirus disease 2019 (COVID-19). Endocr J 2021; 68:129–136. [DOI] [PubMed] [Google Scholar]
- 41.Bennedbæk FN, Gram J, Hegedüs L. The transition of subacute thyroiditis to Graves’ disease as evidenced by diagnostic imaging. Thyroid 1996; 6:457–459. [DOI] [PubMed] [Google Scholar]
- 42.Van den Berghe G. Nonthyroidal illness in the ICU: a syndrome with different faces. Thyroid 2014; 24:1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han T, Kang J, Li G, et al. Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann Transl Med 2020; 8:1077.doi: 10.21037/atm-20-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotondi M, Coperchini F, Ricci G, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Investig 2021; 44:1085–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪.Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid 2021; 31:8–11. [DOI] [PubMed] [Google Scholar]; This is the first study evaluating thyroid function tests in hospitalized patiets with SARS-CoV-2 infection. In this retrospective study serum levels of thyroid function parameters in 50 hospitalized patients with SARS-CoV-2 were compared with those of a healthy control group matched for age and sex, and a group of non-COVID-19 pneumonia patients. At admission, 56% (28/50) of hospitalized SARS-CoV-2 positive patients without previous thyroid diseases, showed lower-than-normal values for TSH (<0.3 mIU/L). Patients with SARS-CoV-2 infection had significantly lower serum levels of TSH and total triiodothyronine (T3) as compared both with the healthy subjects and with patients with non-COVID-19 pneumonia, but thyroid function variables normalized during recovery.
- 46.Lania A, Sandri MT, Cellini M, et al. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol 2020; 183:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪.Muller I, Cannavaro D, Dazzi D, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol 2020; 8:739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study examined thyroid function variables in 85 consecutive SARS-CoV-2 patients admitted to an intensive care unit. Patients treated in the same unit during the equivalent time frame in 2019 served as controls. Serum levels of TSH were significantly lower in SARS-CoV-2 patients than in controls, while the levels of free T3 and free T4 were similar, suggesting a combination of thyrotoxicosis due to atypical thyroiditis and nonthyroideal ilness in SARS-CoV-2 patients. The study also indicates that the observed alterations in thyroid function may be reversible.
- 48▪.Khoo B, Tan T, Clarke SA, et al. Thyroid function before, during, and after COVID-19. J Clin Endocrinol Metab 2021; 106:e803–e811. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compare thyroid function variables between 334 patients hospitalized due to with SARS-CoV-2 infection and 122 patients hospitalized due to a clinical suspicion of COVID-19 but with a negative SARS-CoV-2 test. Ad admission, the prevalence of thyroid dysfunction was similar between patients with and without SARS-CoV-2 infection. During the hospital stay, serum levels of TSH and free T4 were significantly lower among patients with SARS-CoV-2, but returned to normal values during recovery.
- 49.Lui DTW, Lee CH, Chow WS, et al. Thyroid dysfunction in relation to immune profile, disease status and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab 2021; 106:e926–e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao W, Guo W, Guo Y, et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Investig 2021; 44:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou R, Wu C, Zhang S, et al. Euthyroid sick syndrome in patients with COVID-19. Front Endocrinol 2020; 11:566439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campi I, Bulgarelli I, Dubini A, et al. The spectrum of thyroid function tests during hospitalization for SARS-COV-2 infection. Eur J Endocrinol 2021; 184:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruggeri RM, Campennì A, Siracusa M, et al. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones 2021; 20:219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brancatella A, Ricci D, Cappellani D, et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J Clin Endocrinol Metab 2020; 105:dgaa537.doi: 10.1210/clinem/dgaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattar SAM, Koh SJQ, Rama Chandran S, Cherng BPZ. Subacute thyroiditis associated with COVID-19. BMJ Case Rep 2020; 13:e237336.doi: 10.1136/bcr-2020-237336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuno S, Inaba H, Kobayashi KI, et al. A case of postpartum thyroiditis following SARS-CoV-2 infection. Endocr J 2021; 68:371–374. [DOI] [PubMed] [Google Scholar]
- 57.Harris A, Al Mushref M. Graves’ thyrotoxicosis following SARS-CoV-2 infection. AACE Clin Case Rep 2021; 7:14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiménez-Blanco S, Pla-Peris B, Marazuela M. COVID-19: a cause of recurrent Graves’ hyperthyroidism? J Endocrinol Invest 2021; 44:387–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mateu-Salat M, Urgell E, Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID-19. J Endocrinol Investig 2020; 43:1527–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandt F, Thvilum M, Almind D, et al. Graves’ disease and toxic nodular goiter are both associated with increased mortality but differ with respect to the cause of death: a Danish population-based register study. Thyroid 2013; 23:408–413. [DOI] [PubMed] [Google Scholar]
- 61.Lillevang-Johansen M, Abrahamsen B, Jorgensen HL, et al. Excess mortality in treated and untreated hyperthyroidism is related to cumulative periods of low serum TSH. J Clin Endocrinol Metab 2017; 102:2301–2309. [DOI] [PubMed] [Google Scholar]
- 62.Lillevang-Johansen M, Abrahamsen B, Jorgensen HL, et al. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid 2018; 28:566–574. [DOI] [PubMed] [Google Scholar]