Figure 6.
Visual pathway reconstruction with retinal, thalamic, and cortical organoids
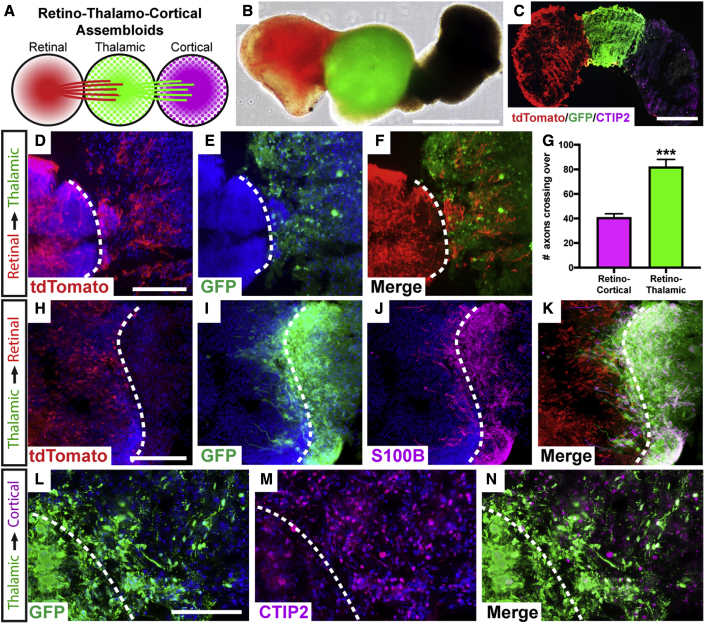
(A) Schematic of strategy to generate tri-assembloids from retinal, thalamic, and cortical organoids.
(B and C) (B) Fluorescent reporters were used to identify various organoids and their projections, with retinal organoids expressing a POU4F2:tdTomato reporter and thalamic organoids expressing a GFP reporter. (C) Staining of cortical organoids with CTIP2 verified their identity.
(D–F) Following the formation of assembloids, tdTomato-expressing RGC axons robustly extended into GFP-expressing thalamic organoids. (D) tdTomato, (E) GFP, (F) merge.
(G) Significantly greater numbers of RGC axons had extended into thalamic organoids compared with brain organoids at the same time point.
(H–K) After an additional 2 months, GFP-expressing thalamic cells migrated retrogradely into retinal organoids, with these migratory cells expressing the early astrocyte marker S100β. (H) tdTomato, (I) GFP, (J) S100β, (K) merge.
(L–N) Between thalamic and cortical organoids, robust extension of GFP-expressing neurites was observed entering CTIP2-positive cortical organoids. (L) GFP, (M) CTIP2, (N) merge
Error bars represent SEM, ∗∗∗p < 0.005. n = 6 separate differentiation experiments each using the H7 and H9 lines of hESCs and the tiPS5 line of hiPSCs. Scale bars: (B) 800 μm, (C) 400 μm, (D–F) 200 μm, (H–K) 150 μm, (L–N) 150 μm. Dashed line indicates boundary between different organoids within assembloid, as indicated on the left margin.