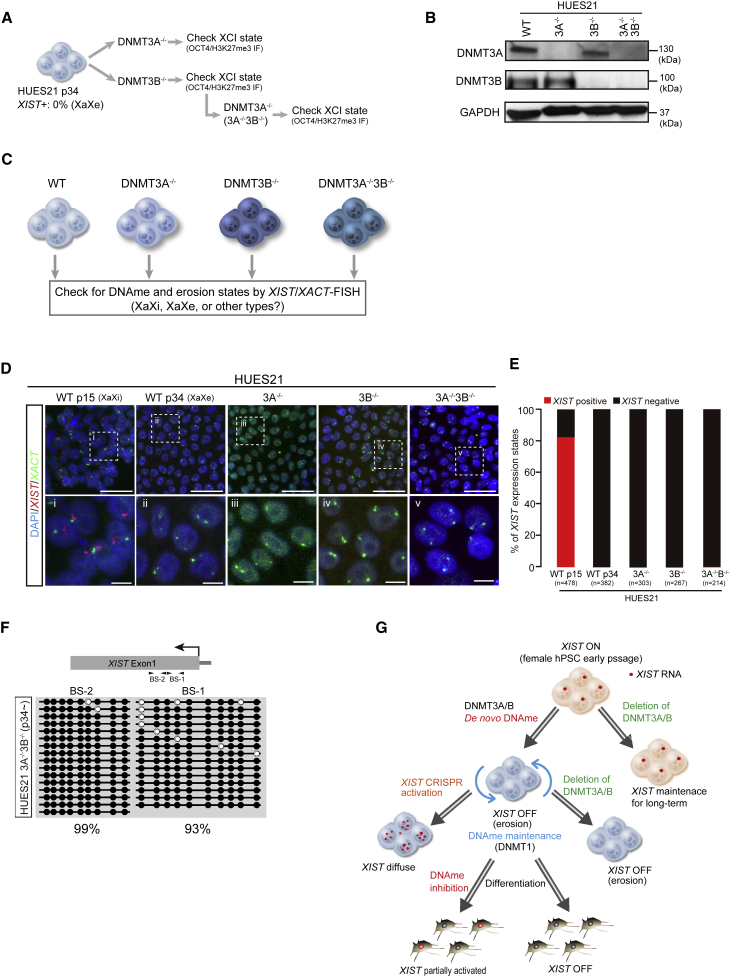
Figure 4.
DNMT3A/3B deletion does not rescue XIST expression after dosage compensation erosion
(A) Experimental scheme for the generation of DNMT3A/3B knockout lines. HUES21 p34 (XIST+ 0%) was used as the parental line.
(B) Western blotting for DNMT3A and DNMT3B. GAPDH was used as the loading control.
(C) Experimental scheme to examine the effect of deletion of de novo DNA methyltransferases on eroded lines.
(D) XIST/XACT RNA-FISH assay. Scale bars represent 10 μm.
(E) Quantification of XIST/XACT expression state. n means the number of cells analyzed. A detailed classification of the results is shown in Figure S4C. Two independent experiments were conducted.
(F) DNA methylation state at XIST promoter regions in DNMT3A−/−3B−/− generated using eroded HUES21 p34.
(G) Model of XIST silencing to initiate erosion in female hPSCs. De novo DNA methyltransferases methylate XIST to silence its expression. Once XIST is silenced, deletion of DNMT3A/3B cannot reactivate XIST and the states are stably maintained, likely by DNMT1. During differentiation, demethylation can partially reactivate XIST, but forced activation of endogenous XIST in eroded hPSCs generates diffused transcripts that cannot act to cause heterochromatization.