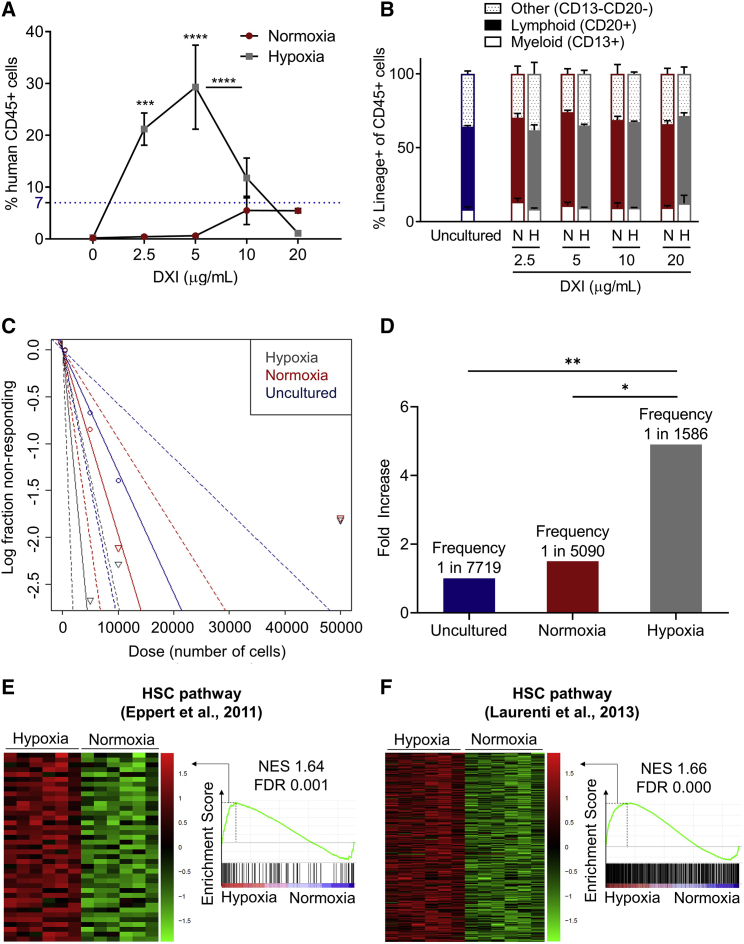
Figure 3.
Concomitant activation of NOTCH and hypoxia pathways enhances ex vivo expansion of human LTR-HSCs
(A) Percentages of human CD45+ cells in the BM of NSG mice 4 months after transplantation of 1 × 105 uncultured CD34+ cells (blue horizontal dotted line) or the 21-day expanded progeny of the same starting cell number for normoxic and hypoxic culture conditions in the absence or with increasing concentrations of DXI (n = 3–4 mice/group).
(B) Lineage distribution of human CD45+ cells in the BM of NSG mice. N, normoxia; H, hypoxia (n = 3–4 mice/group).
(C) Semilogarithmic plot of LTR-HSC frequency 4 months after transplantation of uncultured CD34+ cells or the 21-day expanded progeny of the same starting cell number for normoxic or hypoxic culture conditions in the presence of optimized concentrations of DXI. Solid lines indicate the best-fit linear model for each dataset. Dotted lines represent 95% confidence intervals.
(D) Summary of LTR-HSC frequency and fold increase in LTR-HSC frequency after 21 days of expansion in normoxia or hypoxia with optimized concentrations of DXI relative to uncultured CD34+ cells. p values were measured by extreme limiting dilution analysis.
(E and F) Heatmaps of leading edge subset (left) and enrichment plots (right) showing the relative expression of genes associated with HSC pathways (E, Eppert et al., 2011; F, Laurenti et al., 2013) in CD34+ cells cultured in normoxia or hypoxia with optimized densities of DXI. NES, normalized enrichment score; FDR, false discovery rate.
In (A) and (B), data are displayed as mean ± SEM. Two-way ANOVA was used unless otherwise specified. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001. See also Table S2.