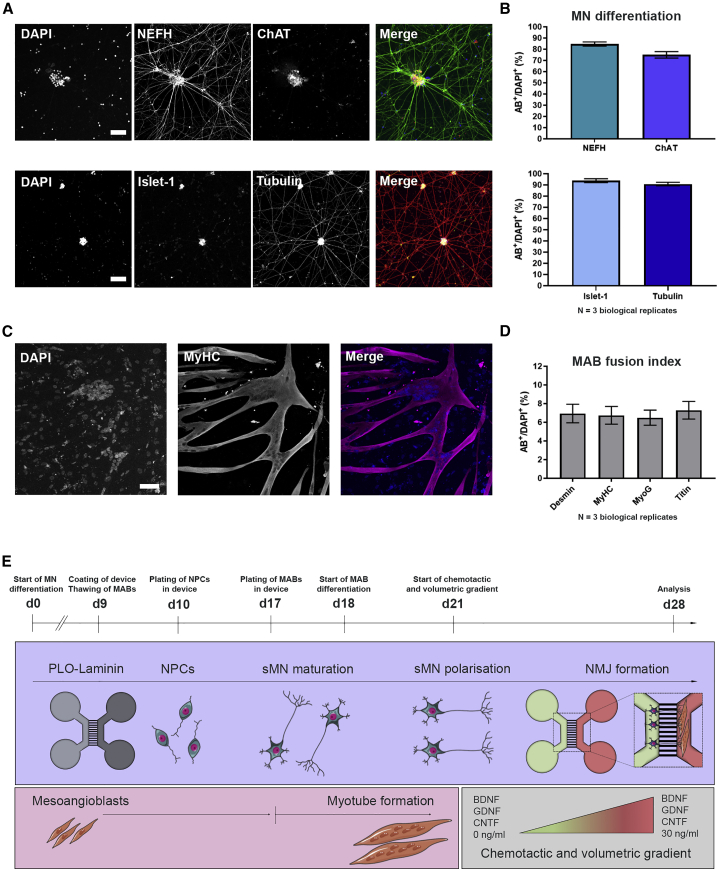
Figure 1.
Characterization of monocultures and overview of NMJ protocol
(A) Confocal images of MNs stained with MN markers neurofilament heavy chain (NEFH), choline acetyltransferase (ChAT), and Islet-1, as well as the pan-neuronal marker βIII-tubulin (Tubulin) at day 28 of MN differentiation. Nuclei stained with DAPI. Scale bars, 75 μm.
(B) Number of cells positive for MN and pan-neuronal markers (AB+). Mean ± SEM of three biological replicates.
(C) Confocal images of myotube heavy chain (MyHC)-positive myotubes 10 days after initiation of differentiation. Scale bar, 75 μm.
(D) Quantification of mesoangioblast (MAB) fusion into multinucleated myotubes (fusion index) with myotube markers desmin, MyHC, myogenin (MyoG) or titin. Mean ± SEM of three biological replicates.
(E) Schematic overview of co-culture protocol and differentiation timeline (days 0–28). Day 0 (d0), differentiation of iPSCs into MN. Day 9 (d9), microfluidic devices are coated with poly-L-ornithine (PLO) and laminin, and MABs are thawed for expansion. Day 10 (d10), MN-NPCs are plated on one side (light gray) of the device. Day 17 (d17), MABs are seeded in the opposite side of the device (dark gray). Myotube differentiation is initiated (d18). Day 21 (d21), a volumetric and chemotactic gradient of neurotrophic factors (BDNF, GDNF and CNTF) is implemented to facilitate polarized growth of spinal MNs (sMN) through the microgrooves toward the myotubes to initiate the formation of NMJs (d28). Cell illustrations were modified from Smart Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
See also Figure S1.