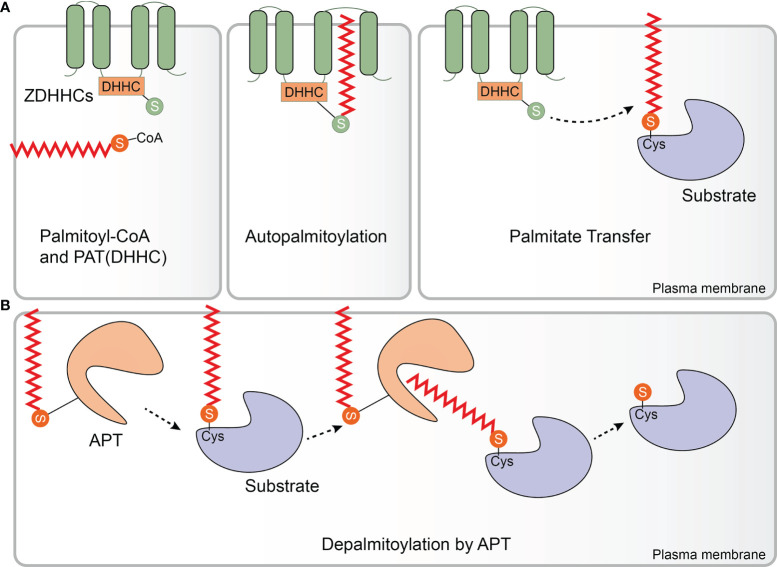
Figure 1.
Protein palmitoylation and depalmitoylation. (A) Palmitate (from palmitoyl-coenzyme A (CoA)) can be attached to substrate proteins via a thioester linkage by Zinc finger aspartic acid-histidine-histidine-cysteine domain-containing protein (zDHHC)-palmitoyl acyltransferases (PATs). zDHHC-PATs are integral membrane proteins (green) that typically have 4 transmembrane domains. The enzyme’s catalytic aspartic acid-histidine-histidine-cysteine (DHHC) motif is located in the cytoplasm. zDHHC-PATs first undergo an autopalmitoylation event before the palmitate group is transferred from the cysteine residue of the DHHC motif to a specific cysteine on a substrate protein (purple). (B) Enzymatic removal of the fatty acid groups from palmitoylated proteins (purple) is catalyzed by acyl protein thioesterases (APTs), a family of serine hydrolases including the enzymes APT1/2 (orange). APT1/2 are themselves palmitoylated which anchors them to the membrane giving them ready access to the fatty acid which is to be removed from the substrate protein. APT1/2 both have a hydrophobic pocket in which the palmitate group binds prior to its cleavage. Following hydrolysis of the thioester bond, the fatty acid is released from the substrate and diffuses into the membrane. (Palmitate is the approximate height of one leaflet of the lipid membrane, here it is enlarged to highlight.).