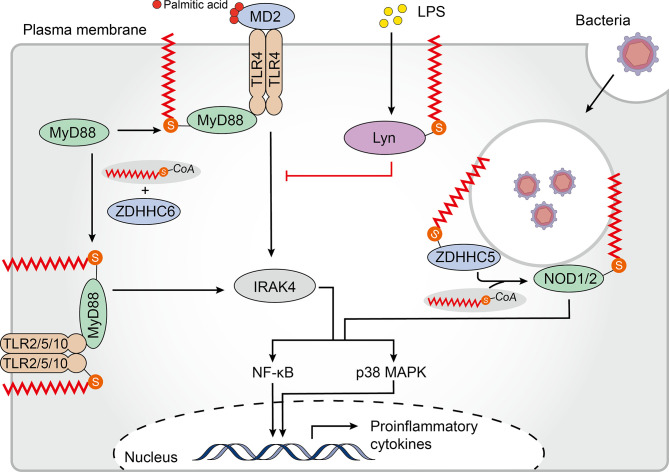
Figure 2.
Palmitoylation in the TLR and NLR pathways. Membrane-bound Toll-like receptors (TLRs)-2/5/10 are all palmitoylated. Palmitoylation of TLR2 targets it to the plasma membrane where it interacts with myeloid differentiation primary response protein (MyD88), which regulates the nuclear factor kappa B (NF-κB) and p38-mitogen-activated protein kinase (MAPK) signaling pathways. Binding of MyD88 to interleukin-1 receptor-associated kinase 4 (IRAK4) and downstream signal activation requires palmitoylation of MyD88 by Zinc finger aspartic acid-histidine-histidine-cysteine domain-containing protein (zDHHC) 6. Although TLR4 is not palmitoylated, palmitic acid binds to its accessory protein myeloid differentiation protein 2 (MD2) to promote pro-inflammatory signaling. Furthermore, lipopolysaccharide (LPS) induces palmitoylation of Lyn kinase, which downregulates TLR4 pro-inflammatory signaling. Palmitoylation of cytosolic nucleotide oligomerization domain-like receptors 1 and 2 (NOD1/2) drives their translocation to bacteria-containing endosomes where they function in signal transduction. zDHHC5 is also recruited to the endosomes that contain bacteria where it palmitoylates both NOD 1 and 2.