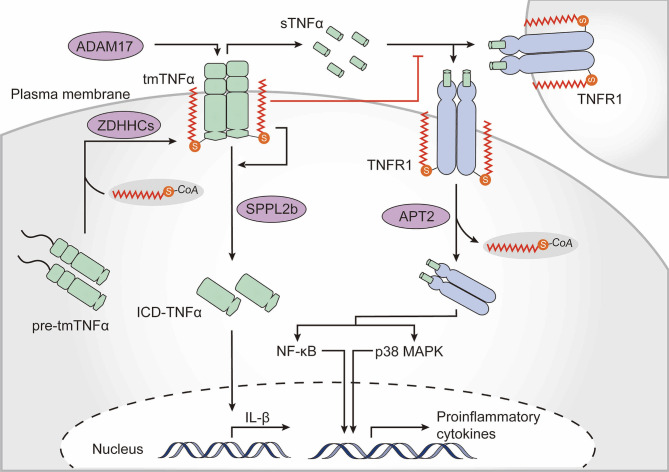
Figure 4.
The role of palmitoylation in the TNFα-TNFR1 signaling pathway. Tumor necrosis factor α (TNFα) is palmitoylated at the boundary between its transmembrane and cytoplasmic domains, which is required for transmembrane TNFα (tmTNFα) localization to lipid rafts. After the extracellular domain of tmTNFα has been cleaved by the TNFα-converting enzyme disintegrin and metalloproteinase domain-containing protein 17 (ADAM17) and soluble TNFα (sTNFα) has been released, the palmitoylated raft-residing tmTNFα is further cleaved by signal peptide peptidase-like 2b (SPPL2b) to generate the intracellular domain of TNFα (ICD-TNFα), which activates the promoter of interleukin (IL)-1β. Binding of sTNFα to TNF receptor (TNFR) 1, however, is blocked by TNFα palmitoylation. TNFR1 also undergoes palmitoylation, which is required for localization within the plasma membrane. Upon sTNFα binding, TNFR1 is de-palmitoylated by acyl protein thioesterase 2 (APT2), which ultimately results in activation of nuclear factor kappa B (NF-κB) and p38-mitogen-activated protein kinase (MAPK).