Abstract
Parkinson’s disease (PD) is a brain disorder that primarily affects motor function, leading to slow movement, tremor, and stiffness, as well as postural instability and difficulty with walking/balance. The severity of PD motor impairments is clinically assessed by part III of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), a universally-accepted rating scale. However, experts often disagree on the exact scoring of individuals. In the presence of label noise, training a machine learning model using only scores from a single rater may introduce bias, while training models with multiple noisy ratings is a challenging task due to the inter-rater variabilities. In this paper, we introduce an ordinal focal neural network to estimate the MDS-UPDRS scores from input videos, to leverage the ordinal nature of MDS-UPDRS scores and combat class imbalance. To handle multiple noisy labels per exam, the training of the network is regularized via rater confusion estimation (RCE), which encodes the rating habits and skills of raters via a confusion matrix. We apply our pipeline to estimate MDS-UPDRS test scores from their video recordings including gait (with multiple Raters, R = 3) and finger tapping scores (single rater). On a sizable clinical dataset for the gait test (N = 55), we obtained a classification accuracy of 72% with majority vote as ground-truth, and an accuracy of ∼84% of our model predicting at least one of the raters’ scores. Our work demonstrates how computer-assisted technologies can be used to track patients and their motor impairments, even when there is uncertainty in the clinical ratings. The latest version of the code will be available at https://github.com/mlu355/PD-Motor-Severity-Estimation.
Keywords: Movement Disorder Society Unified Parkinson’s Disease Rating Scale, Uncertainty, Gait Analysis, Finger Tapping, Computer Vision
Graphical Abstract
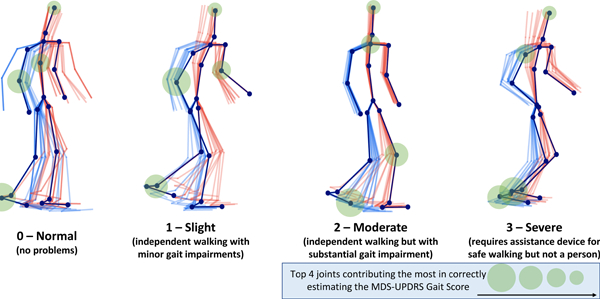
1. Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease (Lang and Lozano, 1998; Ellis et al., 2011). It affects motor function and commonly causes slowing of movements (bradykinesia) and stiffness (rigidity). PD is caused by a gradual decline in dopamine production, resulting in progressive deterioration of selective brain neurons (Lang and Lozano, 1998; Mao et al., 2020; Napier et al., 2020). The degeneration of dopamine-containing cells in the basal ganglia regions provoke visible signs of gait disturbances and postural instabilities (Benatru et al., 2008). Early PD diagnosis and tracking of its signs are crucial for the development and maintenance of treatment plans (Venuto et al., 2016).
In recent years, several methods were investigated for automatic quantification of gait-related PD signs (e.g., gait disturbances and postural instability) using expensive and intrusive wearable sensors (Hobert et al., 2019; Hssayeni et al., 2019; Daneault et al., 2021; Marcante et al., 2021). Alternatively, video technology offers a contactless, scalable, non-intrusive platform for identifying, quantifying, and tracking movement disturbances. Using deep learning algorithms (LeCun et al., 2015) and high performance computing technologies, video processing techniques can now accurately quantify human movements (Kanazawa et al., 2018; Chiu et al., 2019; Kocabas et al., 2020; Adeli et al., 2020). These approaches have yet to be applied to the clinical setting, such as for PD diagnosis.
The Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) (Goetz et al., 2008) defines the most commonly used clinical scoring scheme (Martínez-Martín et al., 2015) to measure the severity and progression of PD. We argue that video of participants performing these guided tests can be used for automatic quantification of PD severity. MDS-UPDRS contains several tests measuring different aspects of movement, including a gait test and a finger tapping test. The MDS-UPDRS gait test requires a participant to walk approximately 10 meters away from and toward an examiner. Trained specialists assess the participant’s posture with respect to movement and balance (e.g., ‘stride amplitude/speed,’ ‘height of foot lift,’ ‘heel strike during walking,’ ‘turning,’ and ‘arm swing’) by observation. The score ranges from 0 indicating no motor impairments to 4 for patients unable to move independently (see Fig. 1). For the MDS-UPDRS finger tapping tests, the participants are asked to tap their index finger to their thumb 10 times as quickly and as big as possible. It is used to measure the motor speed and amplitude of the index finger as a subtle and indirect estimate of cortical motor areas integrity. The finger tapping test is a good indicator of PD since it evaluates bradykinesia, focusing on decrement in rate, amplitude, or both with repetitive action. MDS-UPDRS tests provide principled and well-defined platforms for quantifying PD motor severity.
Figure 1:
Progressive PD impairments demonstrated by 3D gait (poses fade over time; left/right distinguished by color) with MDS-UPDRS gait score shown below each skeleton. Participants are taken from our clinical dataset. Scores 0 to 2 progressively decrease in mobility with reduced arm swing and range of pedal motion (i.e.reduced stride amplitude and footlift) while 3 becomes imbalanced with almost frozen gait. The average movements are the outputs of our proposed pipeline. The top four joints which contributed to gait score estimation are indicated.
Videos of these tests can be automatically processed to quantify movement-linked disease markers. Although there exist a few video-based methods for assessing gait for PD diagnosis (Cho et al., 2009; Xue et al., 2018; Han et al., 2006; Sabo et al., 2020; Fabbri et al., 2020; Stricker et al., 2021), no prior work quantifies movement-linked impairments for assessing PD severity on universally-accepted scales (such as MDS-UPDRS). In a preliminary work (Lu et al., 2020), we used videos of participants performing the maneuvers of the MDS-UPDRS and defined a new task and a principled benchmark by estimating the standard clinical scores. However, one of the major challenges was the possible subjective opinions of clinical raters (rating participants using the MDS-UPDRS scale), which may in turn bias the model to rating habits or subjective opinion of a single rater.
To avoid such challenges, we propose a method to leverage ratings from multiple expert neurologists (3 used herein) to build a robust score estimation model agnostic to single rater habits or preferences. Incorporating ratings from multiple raters introduces a source of uncertainty and noise (Tanno et al., 2019; Nair et al., 2020; Wang et al., 2020; Arteta et al., 2016; Kwon et al., 2019), for which different methods have been presented in the literature. One of the main approaches has been to estimate the skills of raters (or data annotators) while teaching the model to classify the data. The extra knowledge of the rater skills or habits can be crucial information for discovering how confident the ratings from a rater for each input sample should be considered (Long and Hua, 2015). Therefore, accurately modeling the uncertainty (or noise) induced by the ratings of each rater can be useful for improving the accuracy of the final model as well as understanding the quality of the scoring. We have incorporated rating uncertainty concepts into our preliminary work presented at MICCAI 2020 (Lu et al., 2020). Borrowing concepts from (Tanno et al., 2019), we propose a Rater Confusion Estimation (RCE) framework that jointly learns the rater scoring noise and MDS-UPDRS score estimation with the ordinal focal neural network. We do this by creating a learnable confusion matrix (CM) for each rater and optimize for it while classifying the input videos using our ordinal focal strategy (to comply with the ordinal nature of MDS-UPDRS scores and combat small dataset size). We regularize our model within this joint training framework to encourage the estimated raters’ noise to be maximally unreliable, i.e., considering the raters to be maximally uncertain to learn a robust classifier. The model hence learns to discard the unreliable ratings.
Our classification model is applied to the skeletons of the participants extracted from the video. Skeleton extraction uses off-the-shelf models pretrained on large public datasets. The estimation of the MDS-UPDRS scores will only be performed on low-dimensional skeletons (49 keypoints/joints in 3D space for gait and 21 hand keypoints), which anonymizes the data and makes it agnostic to the clinical environment and video background.
In summary, this paper is a novel extension of our earlier work (Lu et al., 2020) with respect to six points: (1) Addition of more participants in the study, primarily of class 0, scored by expert neurologists in place of the public dataset. (2) Novel assessment of clinical inter-rater reliability for the MDS-UPDRS gait test with ratings from three different expert raters. (3) Supplementing our model with the RCE framework to incorporate uncertainty induced by rater disagreements. We added an explicit simplex projection to the RCE training step and used our revised ordinal focal loss function. (4) Visualization of saliency with respect to the contribution of individual body joints to score estimation of MDS-UPDRS scores (green circles in Fig. 1). This approach examines the validity of salient features from the input keypoints by our model to estimate gait or finger tapping scores. (5) Modification of our ordinal-focal (OF) loss function by incorporating a new weighting scheme. (6) Finally, in addition to the MDS-UPDRS gait test, we extend our method to the widely-used finger tapping test, which examines fine motor control of the upper extremities. We present the first automatic vision-based method to predict MDS-UPDRS finger tapping scores.
2. Related Work
PD Severity Estimation.
Prior work aiming to objectively assess PD severity or progression are either based on neuroimages (Adeli et al., 2016; Bharti et al., 2019; Sivaranjini and Sujatha, 2020) or largely rely on quantifying motor impairments via wearable sensors that are expensive, unwieldy, and sometimes intrusive (Hobert et al., 2019; Hssayeni et al., 2019). Video-based technologies based on deep learning now offer non-intrusive and scalable ways to quantify human movements (Kanazawa et al., 2018; Chiu et al., 2019). Their application to the clinical setting, such as PD severity estimation, is under-explored. Video-based approaches for PD assessment, e.g., (Cho et al., 2009; Xue et al., 2018; Han et al., 2006; Sabo et al., 2020; Fabbri et al., 2020; Stricker et al., 2021), have mainly focused on diagnosis of PD (a binary classification) rather than quantifying motor impairment severity in a principled or standardized way.
Cognitive Assessment from Motor Movement.
General cognitive assessment from motor movement with vision-based approaches has included the use of deep neural models to predict cognitive dysfunctions such as Executive Function Disorder and Alzheimer’s disease (Babu et al., 2019; Gattupalli et al., 2017; Chandra et al., 2015). Upper-body gesture recognition from the Praxis test (Chandra et al., 2015; Heilman et al., 1982), a gesture-based diagnostic for neurological pathologies such as Alzheimer’s disease which measures the ability of patients with no motor or sensory deficit to plan and perform skilled movements, is used in (Negin et al., 2018) to perform automatic cognitive assessment. This method demonstrated promise in automatically performing cognitive assessment from gestures with various methods such as CNN and coarse skeleton features in large upper-body or hand movements. We extended this to finer finger pinching movements and to the MDS-UPDRS test using recent developments in fine hand keypoint detection (Simon et al., 2017).
Similarly to gait, prior work has used wearables such as accelerometers to predict the finger tapping MDS-UPDRS test (Stamatakis et al., 2013). (Lee et al., 2016) developed a smartphone-based finger tapping application to assess bradykinesia. Vision-based approaches include early attempts to develop features to represent finger tapping motion involving a polygon masking approach (Criss and McNames, 2011). A concurrent work (Li et al., 2021) developed a complex action recognition approach for assessing finger tapping motor impairments. In another work, (Lin et al., 2020) developed three features to assess bradykinesia in single RGB videos using the MDS-UPDRS hand movement test.
Label Uncertainty.
Label noise can greatly affect the efficacy of machine and deep learning models, especially in medical applications, which often have small datasets, require domain expertise and suffer from high inter-rater and intra-rater variability (Karimi et al., 2020). Many techniques have been explored to handle this variability, such as label cleaning (Pham et al., 2019) and changes in network architecture changes (Dgani et al., 2018). Numerous studies have proposed to keep the initial dataset, modeling, and training methods intact while only changing the loss function, such as in (Ghosh et al., 2017; Wang et al., 2019; Izadinia et al., 2015). Specifically focusing on the case with scores from multiple medical experts, Tanno et al. (Tanno et al., 2019) proposed an annotator confusion estimation method, which learns rater confusion matrices jointly with the correct label during training.
The literature review by Karimi et al. (Karimi et al., 2020) performed a comparison on three medical imaging datasets with different types of label noise. It demonstrated that the medical image analysis community does not share a standard set of guidelines or shared practice regarding how to handle label noise, and “recent progress on handling label noise in deep learning has gone largely unnoticed by the medical image analysis community.” They showed that in the multiple annotator setting (like ours), the rater confusion (Tanno et al., 2019) and Improved Mean Absolute Error (iMAE) (Wang et al., 2019) methods achieve highest performance.
3. Materials and Methods
3.1. Participants
We collected video recordings of MDS-UPDRS exams from 55 participants which were scored by three different board-certified movement disorders neurologists (referred to by raters A, B, and C). The PD patients met UK Brain Bank diagnostic criteria (Clarke et al., 2016). All procedures were approved by the Stanford Institutional Review Board and written informed consent was obtained from all participants in this study, which prohibits public release. This dataset is an extension of the one in (Lu et al., 2020), which was confined to 30 gait videos rated by Expert A. We refer to the old ratings by A*. Rater A re-scored all 55 exams after over 1 year. All raters rated the gait videos based on the MDS-UPDRS section 3.10. The ground-truth score of a video (0, 1, 2, or 3) was determined based on the majority vote among the three raters, with ties randomly broken. The inter-rater agreement is further visualized in Fig. 2. Since A* ratings were performed before additional videos were added, videos not scored are left blank in the table. Videos of PD participants were recorded during the off-medication state, defined according to previously published protocols (Poston et al., 2016). We first extracted the sections of the video documenting the gait examination, in which participants were instructed to walk directly toward and away from the camera twice. The gait clips range from 5 seconds to 54 seconds with 30 frames per second. Score distribution and related information of the 55 gait exams are provided in Table 1.
Figure 2:
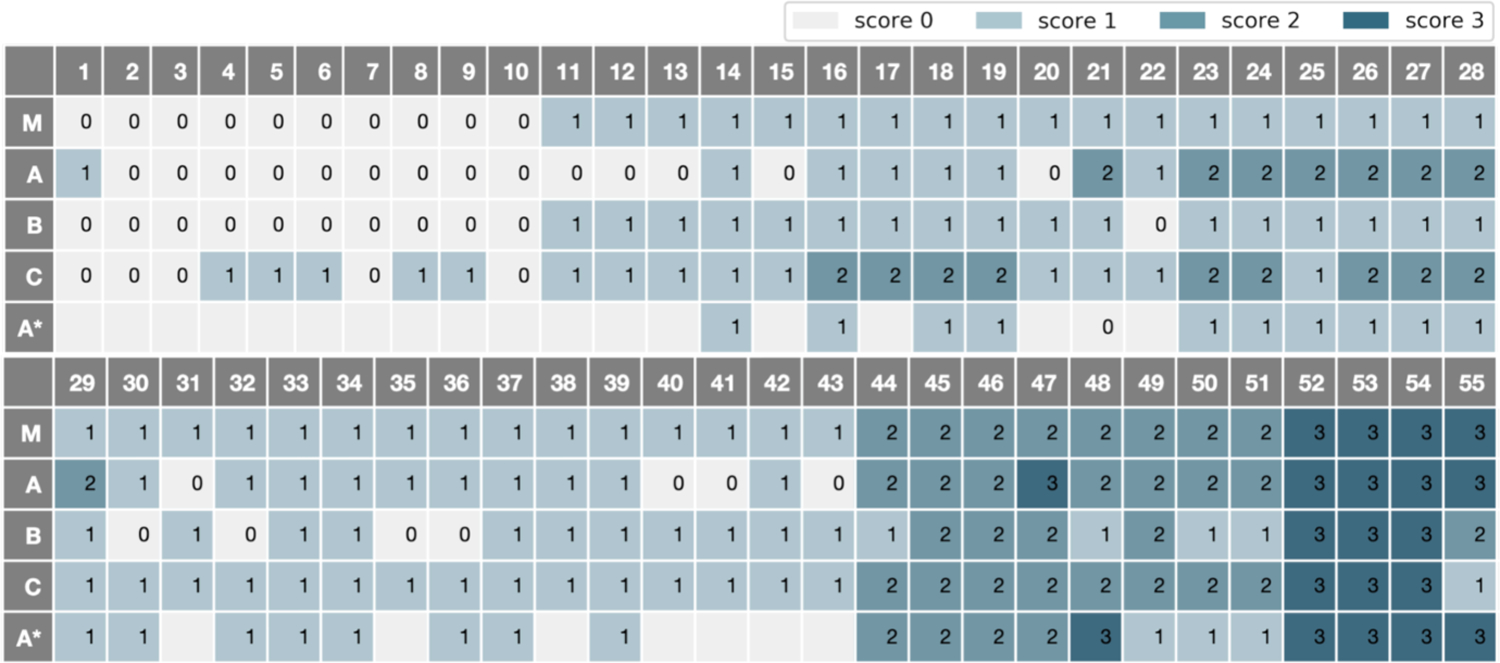
Gait Scores of Raters A, B, C, A* and their Majority Vote (M).
Table 1:
Clinical dataset used in this study. Ground-truth for each video is determined by the majority vote of raters (ties randomly broken). Walking time denotes the length of the gait exam in seconds and Finger tapping time refers to the length of the finger tapping exam.
| MDS-UPDRS Score | All | 0 | 1 | 2 | 3 | |
|---|---|---|---|---|---|---|
| Gait | N | 55 | 10 | 33 | 8 | 4 |
| Sex (F/M) % | 49/51 | 70/30 | 42/58 | 50/50 | 50/50 | |
| Age (mean ± std) | 61.2± 14.9 | 46.9± 16.3 | 62.2± 13.1 | 68.1± 8.3 | 75.8± 1.3 | |
| Walking Time (sec) | 20.9±13.7 | 7.3± 2.04 | 20.7± 12.5 | 30.0± 11.2 | 38.0 ± 10.8 | |
|
| ||||||
| Right Hand | N | 34 | 3 | 11 | 10 | 10 |
| Sex (F/M) % | 41/59 | 67/33 | 36/64 | 50/50 | 30/70 | |
| Age (mean ± std) | 69.7± 7.3 | 73.2± 10.1 | 66.7± 7.6 | 69.8± 7.1 | 71.9± 4.3 | |
| Finger Tapping Time (sec) | 12.9± 5.0 | 9.5± 2.9 | 13.4±3.3 | 13.5±6.3 | 12.6±5.2 | |
|
| ||||||
| Left Hand | N | 34 | 2 | 10 | 12 | 10 |
| Sex (F/M) % | 41/59 | 0/100 | 60/40 | 58/42 | 10/90 | |
| Age (mean ± std) | 69.7± 7.3 | 70.5± 11.5 | 68.7± 5.2 | 69.5± 8.5 | 70.8± 6.1 | |
| Finger Tapping Time (sec) | 10.2±3.2 | 8.5±2.9 | 9.2±2.8 | 10.1±2.8 | 11.6±3.4 | |
We further extracted the sections of the participant videos documenting the finger tapping examination, in which participants were instructed to tap the index finger on the thumb 10 times as quickly and as big as possible. Each hand was rated separately in evaluating speed, amplitude, hesitations, halts, and decrementing amplitude. These videos were scored by a single rater based on the MDS-UPDRS section 3.4. The finger tapping clips range from 4 seconds to 30 seconds with 30 frames per second. Our dataset includes 34 exams each for right and left finger tapping, for a total of 68 exams with 5 of score 0, 21 with score 1, 22 with score 2, 17 with score 3 and 3 with score 4. Details of these participants are also provided in Table 1.
In both gait and finger tapping experiments, participants who cannot perform the test at all or without assistance are scored 4. We combined scores 3 and 4 due to limited number of videos scored 4. As can be seen in Table 1, participants are relatively age- and sex-matched across scores in the finger tapping experiment. In the gait experiments, older participants are more likely to be scored higher on the scale (more severe). Due to patient privacy constraints we are not allowed to release the videos or the dataset.
3.2. Estimation of MDS-UPDRS Scores from Videos
As shown in Fig. 3, a monocular video of the participant walking in the scene forms the input. Our method consists of two main steps: 3D skeleton extraction and motor score estimation.
Figure 3:

The proposed framework: we first extract the identified participants’ 3D body mesh and subsequently the skeletons via VIBE. Based on this 3D skeleton sequence, our proposed OF-DDNet estimates the MDS-UPDRS gait score.
3.2.1. Skeleton Extraction
Gait
We extract the 3D skeleton from the gait videos with VIBE (Video Inference for human Body pose and shape Estimation) (Kocabas et al., 2020). This is an extension of SPIN (SMPL oPtimization IN the loop) that we previously used in (Lu et al., 2020), a state-of-the-art neural method for estimating 3D human skeleton and shape from 2D monocular images of an input video. It is initialized with pretrained SMPL (Loper et al., 2015). The pipeline first recovers the 3D body mesh using Human Mesh Recovery (HMR) (Kanazawa et al., 2018) pretrained on the large publicly-available Human3.6M (Ionescu et al., 2013) and MPI-INF-3DHP (Mehta et al., 2017) datasets, providing over 150k training images with 3D joint annotations, as well as large-scale datasets with 2D annotations (e.g., COCO (Lin et al., 2017) and MPII (Andriluka et al., 2014)). In summary, this process reduces the videos to 3D human meshes and regresses them to skeletons with 49 predefined joints, as in (Ionescu et al., 2013).
Hand
We additionally extract 2D hand skeleton from the finger tapping videos with the OpenPose hand keypoint detection system (Simon et al., 2017), which produces 21 keypoints for each of the right and left hands. This system uses a training process called multiview bootstrapping with multiple cameras to produce fine-grained detectors for hand keypoints with greater robustness to noise and occlusion. A keypoint detector is first initialized to produce noisy scores in multiple views of the hand, which are triangulated in 3D and the reprojected to be used iteratively as new scores during training. We select this hand detection model for our finger tapping experiment because it is a single-view image-based hand keypoint detector comparable to methods that use depth sensors. It produces numerous and precise hand keypoints that are crucial to representing fine hand movements and hence quantify PD motor impairments.
3.2.2. Score Estimation from Skeletons Based on Single Rater Scores
We first review our model, OF-DDNet, proposed in our prior work (Lu et al., 2020). The model architecture is based on the Double-Feature Double-Motion Network (DD-Net) (Yang et al., 2019). OF-DDNet uses features extracted from skeletons (for both gait and finger tapping exams) to estimate the MDS-UPDRS scores, whose ground-truth is determined by one single rater.
Feature Construction
We transform the skeleton data and their movement over time in a video clip into a series of features as the input to the classification model. We use two types of features to address the variance of 3D Cartesian joints to both location and viewpoint: (1) Joint Collection Distances (JCD) and (2) two-scale motion features. Let be the 3D Cartesian coordinates of the jth joint at frame t, where j ∈ {1,…,n} and t ∈ {1,…,T}. JCD is then defined as a location-viewpoint invariant feature that represents the Euclidean distances between joints as a matrix M, where for joints j and k at frame t. Since M is a symmetric matrix, only the upper triangular matrix is preserved and flattened to a dimension of for n joints. A two-scale motion feature is introduced for global scale invariance which measures temporal difference between nearby frames. To capture varying scales of global motion, we calculate slow motion and fast motion
| (1) |
where denotes the set of joints for the tth frame. The JCD and two-scale motion features are embedded into latent vectors at each frame through a series of convolutions to learn joint correlation and reduce the effect of skeleton noise. Then, for the ith video clip, the embeddings are concatenated and run through a series of 1D temporal convolutions and pooling layers, culminating with a softmax activation on the final layer to output a probability for each of the C classes pi = [pi,1,…,pi,C]T (see Fig. 3). Due to our relatively small clinical dataset compared to large deep learning datasets, we choose a lightweight architecture with temporal CNNs to prevent overfitting.
Hybrid Ordinal-Focal (OF) Loss
We leverage the ordinal nature of MDS-UPDRS scores to combat the natural class imbalance in clinical dataset by proposing a hybrid ordinal (O) focal (F) loss.
One of the use cases of the focal loss (Lin et al., 2017) has been to combat class imbalance. It was initially proposed for binary classification, but it is naturally extensible to multi-class classification (e.g., C > 2 classes). Let yi = [yi,1,…,yi,C] be the one-hot-encoding label for the score of the ith training sample. The focal loss is then defined as . The modulating factor (1 − pi,c)γ is small for easy negatives where the model has high certainty and close to 1 for misclassified examples. This combats class imbalance by down-weighting learning for easy negatives, while preserving basic cross-entropy loss for misclassified examples. We set the default focusing parameter of γ = 2 and weighting factor α = 0.25 as suggested by (Lin et al., 2017).
To leverage the intrinsic order in the MDS-UPDRS scores, we propose to use an ordinal loss, which penalizes predictions more if they are violating the order. Let yi = argmaxc{yi,c} be the actual score for the ith video (yi ∈ {0,1,2,3}), and let be the estimated score. We calculate the absolute distance and incorporate this with categorical cross-entropy to generate our ordinal loss .
Finally, ordinal and focal losses can be naturally combined by factorizing them with scaling factor β for the ordinal component as
| (2) |
3.2.3. Multiple Rater Scores
In the presence of uncertainty in the label (score) space (MDS-UPDRS Gait scores provided by three different raters), we propose an extension to the OF loss. We introduce a rater confusion data-weighting and loss scheme inspired by (Tanno et al., 2019). In this framework, we learn both the OF-DDNet model parameters and scoring patterns of each rater, akin to a rater profile. Each scoring profile is encoded as a confusion matrix (CM) utilized by the Rater Confusion Estimation (RCE) technique described in the following.
Assume we have noisy scores from R different raters, where r ∈ {1,…,R} (R is the total number of raters). We construct a confusion matrix A(r) for rater r, where the (c׳,c) entry of the matrix is the probability , i.e., the probability that rater r corrupts the ground-truth score yi = c to . Note, this probability is independent of the input image and solely characterizes the systematic bias of rater r in scoring videos. Assuming pi is the true class probability vector for the ith video estimated by our model, the RCE-weighted prediction is then the estimated class probability vector weighted by the confusion matrix of rater r (see Fig. 4 for details). As such, the true scores and confusion matrices are jointly optimized with our OF loss function
| (3) |
Figure 4:
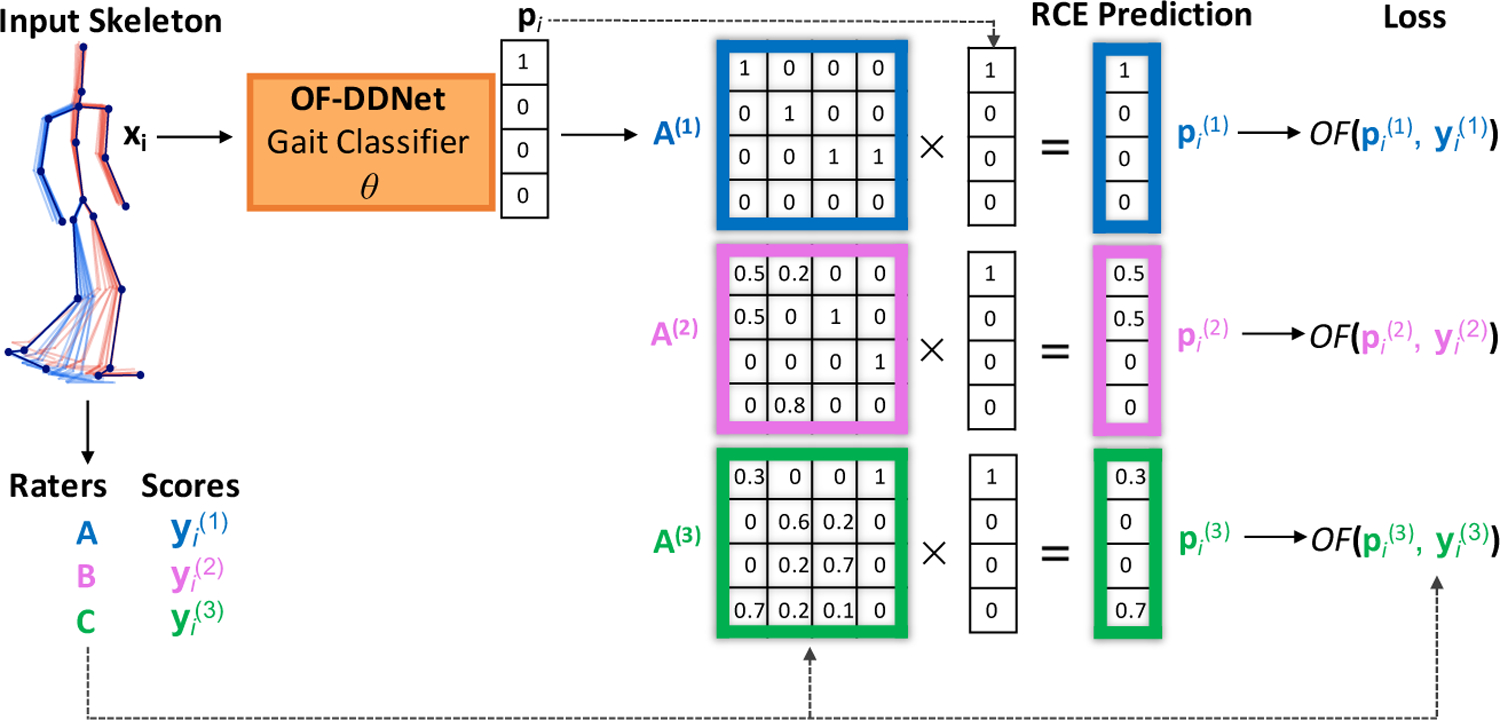
The OF-DDNet training pipeline with Rater Confusion Estimation (RCE). We implemented this scheme for the gait MDS-UPDRS score estimation with 3 raters. Given a series of input skeletons, OF-DDNet generates prediction pi for the input. This prediction is multiplied by each rater CM A(r) to produce a prediction for each rater. The total loss is the sum of the individual losses between each pair of the rth rater.
As suggested in (Tanno et al., 2019), when {A(r)} are initialized as identity matrices, a trace regularization can be used in practice to find the maximal amount of confusion to best explain the noisy observations.
To implement the optimization defined in Eq. (3), we have to ensure that each column of A(r) defines a conditional probability that sums up to 1. We propose to satisfy this constraint by projecting the columns of A(r) onto a simplex in each optimization iteration via an explicit projection operation, similar to the projection operation in (Cabral et al., 2011). To this end, before calculating our loss for each training step, we set
| (4) |
4. Experiments
We preprocess our dataset by first subclipping each video into shorter samples and then normalizing joints per clip after skeleton extraction. The gait exams are subclipped into samples of 100 frames each, creating multiple subclips from each single exam video. The finger tapping clips preserve the end of the exam, due to the nature of the exam requiring counting and observation of pauses or slowing over time. The finger tapping subclips start at multiples of 80 frames offset from the beginning of the exam and finish at the end of the exam. Due to clips containing overlapping components, we add Gaussian noise to each subclip distributed as .
During training, the ground-truth score of each subclip is the same as the score of the exam. During evaluation, the predicted score of an exam is the majority vote among its subclips. This subclipping and subvoting mechanism adds robustness to the overall system and allows us to augment the dataset for proper training of the OF-DDNet. To account for the limited dataset size, all evaluations in this study were performed using a participant-based leave-one-out cross-fold-validation on all samples. The clips and subclips for each exam are never separated by the train/test split.
Note, we used pretrained skeleton estimation models provided by VIBE (Kocabas et al., 2020) and OpenPose (Simon et al., 2017). However, due to the lack of joint-level 3D annotations, we cannot directly evaluate their performance for skeleton estimation from our videos. We evaluate the validity of the extracted skeletons qualitatively as well as through estimating the MDS-UPDRS scores. The extracted skeletons resulted in classification models (for our method and all of the baselines) significantly better than chance (p-value < 0.05 using Wilcoxon signed rank test (Wilcoxon, 1992)).
Setup.
Optimal hyperparameters for both models were obtained by performing a grid search using inner leave-one-out cross validation and the Adam optimizer (β1 = 0.9,β2 = 0.999) (Kingma and Ba, 2014). Note that the clips for each subject are never separated by the train/test split. Leave-one-out cross-validation is performed on a per-subject level, wherein all video clips of exactly one subject are used for testing and removed from the training fold at each iteration. Best performance was achieved at 300 epochs, batch size of 128, filter size of 32 and an annealing learning rate from 1−3 to 1−6. All models were developed with Keras 2.2.4 (Gulli and Pal, 2017) and ran on an Nvidia Tesla K80 GPU with 12GB memory. Each training run in both experiments took approximately 1 to 2 hours.
Evaluation Metrics.
For evaluation, we report per-class and macro average F1, area under ROC curve (AUC), precision (Pre), recall (Rec), and balanced accuracy (bAcc). To assess whether the bAcc is significantly higher than chance, we ran a null classifier 10,000 times, which randomly assigned each sample one of the 4 scores (a probability of 25%). The null distribution was then built for the bACC and one-tailed p-values were derived based on the true bAcc of our model. One-tailed p < 0.05 is used as the significance threshold.
For the gait experiment with multiple raters, the ground-truth score of each exam is the majority vote of the raters. In addition to the above metrics, we use Cohen’s κ coefficient (κ) to measure the agreement between prediction and ground truth. κ is considered a more robust measure compared to the simple percent agreement measure because it considers the possibility that agreement occurs by chance. Lastly, we measure the pairwise Cohen’s κ between the scores provided by two different raters to asses inter-rater reliability (McHugh, 2012).
4.1. Gait Score Estimation Results - Multiple Rater Scores
For this experiment, three different raters scored the videos. We ran our OF-DDNet in conjunction with RCE (explained in Sec. 3.2.3). The Rater Confusion Estimation requires the initialization of the rater CMs as learnable parameters; this was implemented as a final layer on the output of softmax in order to store the CMs as learnable variables.
Our method achieves a κ of 0.49 (Table 2) and macro-average AUC of 0.83, F1-score of 0.66, precision of 0.63, and balanced accuracy (average recall) of 72% (see per-class performance metrics in Fig. 5). Furthermore, the classification performed significantly better than the null classifier based on permutation testing (one-tailed p < 0.0001).
Table 2:
Comparison with baseline and ablated methods.
| Method | κ | AUC | F 1 | Pre | Rec |
|---|---|---|---|---|---|
| Ours | 0.49 | 0.83 | 0.66 | 0.63 | 0.72 |
| 1) Ours w/o Ordinal | 0.46 | 0.83 | 0.67 | 0.69 | 0.72 |
| 2) Ours w/o Focal | 0.46 | 0.84 | 0.66 | 0.64 | 0.70 |
| 3) Ours w/o OF loss* | 0.45 | 0.80 | 0.66 | 0.67 | 0.65 |
|
| |||||
| 4) RCE w/ implicit norm* | 0.41 | 0.77 | 0.63 | 0.65 | 0.61 |
| 5) Soft scores* | 0.32 | 0.75 | 0.56 | 0.57 | 0.56 |
| 6) Soft scores (KL)* | 0.42 | 0.72 | 0.62 | 0.65 | 0.62 |
|
| |||||
| 7) Majority Vote (OF)* | 0.33 | 0.73 | 0.58 | 0.59 | 0.58 |
| 8) Majority Vote* | 0.32 | 0.75 | 0.56 | 0.57 | 0.56 |
|
| |||||
| 9) Baseline OF-CNN* | 0.26 | 0.72 | 0.57 | 0.60 | 0.54 |
| 10) Baseline CNN* | 0.24 | 0.71 | 0.55 | 0.61 | 0.49 |
| 11) DeepRank* ((Pang et al., 2017)) | 0.27 | 0.70 | 0.56 | 0.53 | 0.58 |
| 12) SVM* | 0.21 | 0.56 | 0.44 | 0.49 | 0.40 |
indicates statistical difference at (p < 0.05) compared with our method, measured by the Wilcoxon signed rank test (Wilcoxon, 1992). Best results are in bold and second best are underlined. See text for details about compared methods.
Figure 5:
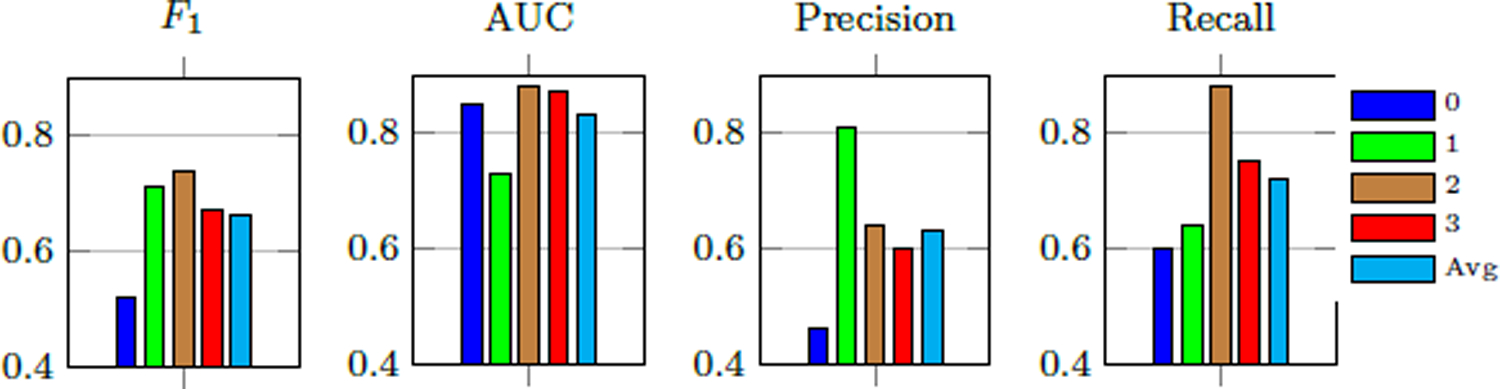
Per-class MDS-UPDRS gait score estimation performance of our method.
As seen in the confusion matrix (Fig. 6), all errors are within one score deviation of the ground-truth (based on majority vote), with the exception of a small portion of score 1 misclassified as score 3. This indicates that our model successfully forms predictions within a range very similar to the raters themselves. This claim is also supported by Fig. 7, where possible ratings for each participant are defined as scores that at least one rater provided for the participant. 46 out of 55 participants were given a score by the model that was also assigned by a physician, with only 9 participants not assigned a score that matches a physician rating. This translates to a 83.7% accuracy that our method rates the participants’ gait exam within the range of actual physician ratings. Classwise, the percent of model predictions within the range of actual physician ratings for classes 0, 1, 2, 3 are respectively 90.9%, 84.8%, 62.5%, and 100% using majority vote as the ground-truth. This demonstrates that most of our model predictions can be considered acceptable scores. Furthermore, we observe from Fig. 5 and Fig. 6 that scores 0 and 1 have the lowest percent accuracy as the model tends to misclassify them as each other. Score 1 has the greatest spread of errors as it has the largest number of participants. The only misclassified exam with score 3 is assigned a different score by all three raters (see participant 55 in Fig. 2).
Figure 6:
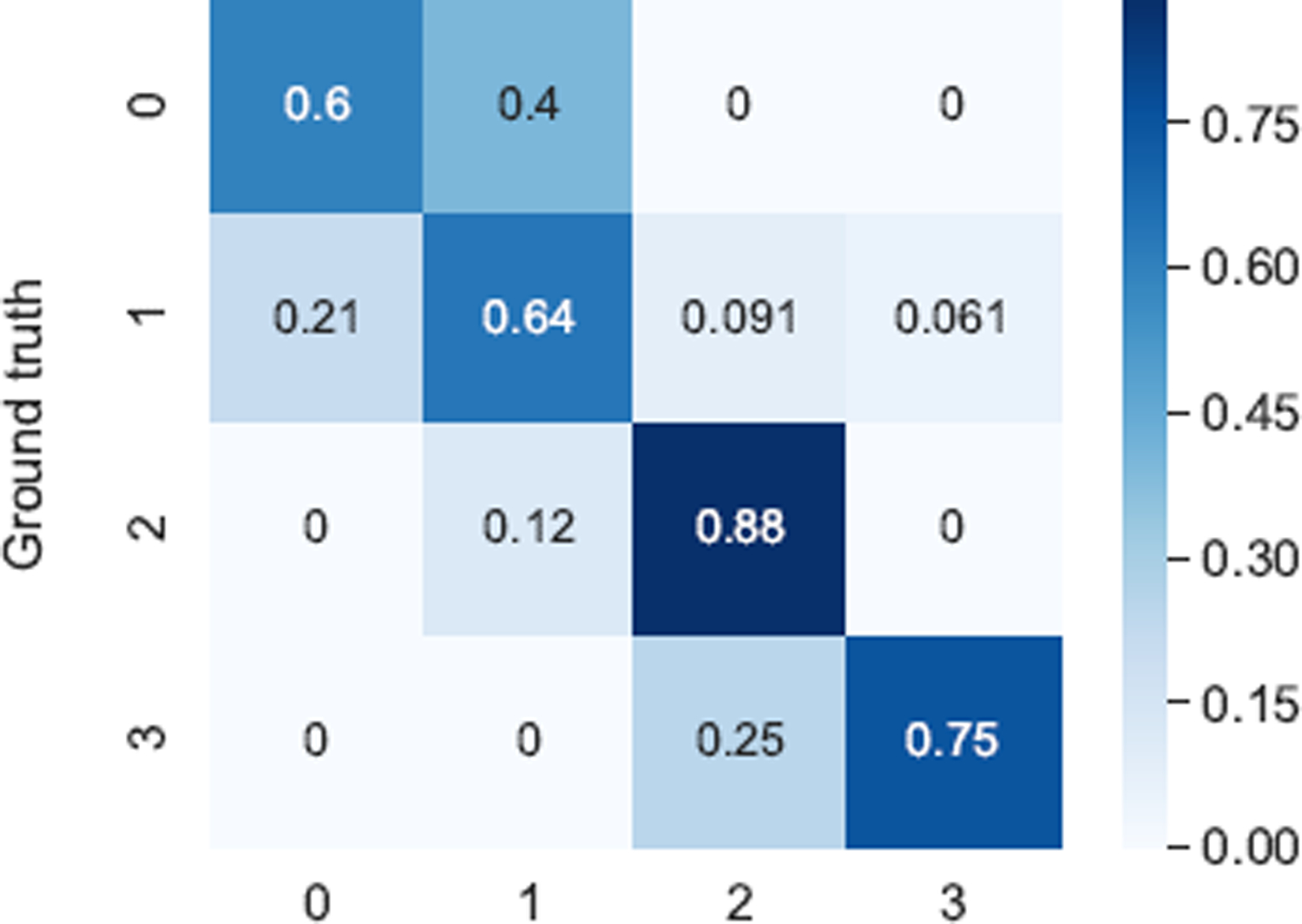
Confusion matrix of our final model for estimation MDS-UPDRS gait scores.
Figure 7:
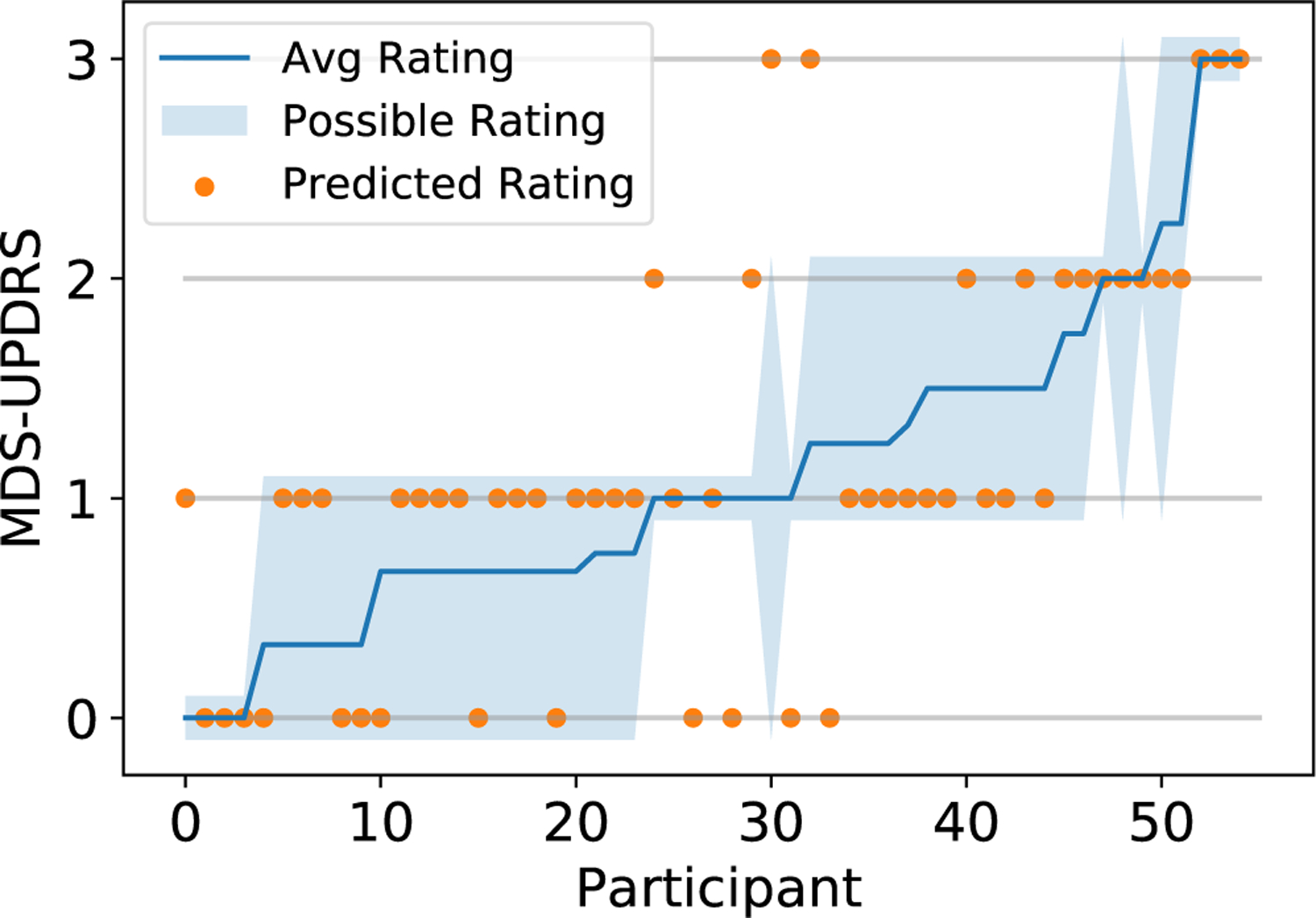
MDS-UPDRS gait score as a function of study participants. Orange dots indicate predicted scores by our method. Possible rating refers to all ratings the specific participant has received from the three raters. The participants are sorted in an increasing average rating order.
Comparison with Baseline Methods and Ablations
We compare our results with several baselines in Table 2. Majority vote of all ratings per exam is taken as ground truth, and all methods use categorical cross-entropy loss unless otherwise specified. We first perform ablations of our method, OF-DDNet with RCE, to handle multiple rating scores:
Our method without ordinal loss (i.e., focal loss with CE);
Our method without focal loss (i.e., ordinal CE loss);
Our method without ordinal-focal loss (i.e., simple multi-class CE loss);
For this comparison, we examine the effect our simplex projection explained in Sec. 3.2.3. We implement RCE with an implicit normalization of the A(r) matrices. Instead of our simplex projection (Eq. (4)), we modify the loss function in Eq. (3) by multiplying the A(r) and by the factor. This operation will incorporate the normalized values in the loss, but does not replace the values in the A(r) matrices. Since we are no longer explicitly setting A(r), the columns are no longer guaranteed to sum to one and may diverge during training;
As an alliterative approach to our proposed RCE OF-DDNet, we examine probabilistic techniques to model uncertainty by representing each label as a probability distribution instead of as a one-hot vector, as traditionally used in cross-entropy loss functions. This technique has been used to combat label noise and has been shown to be effective in cases of multiple raters with high uncertainty (Nguyen et al., 2014). The score for each gait exam xi is represented as a vector Li ∈ ℝC for C classes, where L(c) is the percent of raters who provided a score of c for xi for c ∈ {0,…,C − 1}. This is an extension of CE-loss, which models the uncertainty in the scores and encourages the model to minimize the distance between the estimated distribution and the score distribution. We refer to this method as ‘Soft Scores,’ trained by a CE loss with the DD-Net architecture;
Soft Scores trained by KL divergence loss (instead of CE) with the DD-Net architecture;
Finally, we use the majority vote across all ratings for each exam to train the following models, starting with the original OF-DDNet without RCE;
The baseline DD-Net with CE;
1D CNN modeled after DD-Net architecture sans double features and embedding layer using input of raw 3D joints from VIBE;
the same as (1) but with our OF loss;
DeepRank (Pang et al., 2017), a ranking CNN which cannot be combined with focal loss;
Support Vector Machine (SVM) using the raw 3D joints.
The results of our proposed RCE and OF-DDNet model along with our listed baselines and ablations are summarized in Table 2. First, our method with or without ordinal loss outperform all other methods. Based on the Wilcoxon signed rank test (Wilcoxon, 1992) (p < 0.05), our proposed method achieves significantly better performance than several other methods and consistently outperforms the remaining methods in the table. All metrics were performed on a per subject level except the AUC and the Wilcoxon signed rank test. The latter were performed on video clips, for which our model outputs class probabilities as needed for calculating the AUC. The statistical test results are thus more representative of the data used for training and evaluation of the models. Remarkably, our method also outperforms human raters by showing higher agreement with majority vote compared to inter-rater agreement with regard to average pairwise κ, F1-score, precision and (bAcc)/recall. Our κ of 0.49 is much higher than the κ of all rater pairs, which are 0.38, 0.39, and 0.30 (see Table 3). In addition, RCE improves performance over non-RCE methods by a heavy margin, with the exception of the KL Loss. This suggests that rater confusion estimation works very well for modeling the uncertainty in the scores. We note that a DD-Net model with soft scores achieves higher performance with KL-Divergence than with Categorical Cross Entropy loss. All methods utilizing the score distribution achieve higher performance than majority vote methods, which aggregate the scores into a single rating score before training. Adding focal (Method 1) loss to baseline DD-Net improves κ, F1-score, AUC, and precision; however, adding ordinal (Method 2 in the Table) loss to baseline DD-Net results in higher F1 and equivalent AUC, but lower κ, precision and recall than the baseline RCE. Ordinal loss could be clashing with the baked in ordinality of the rater CMs, which already contain probabilities increasing with distance to the true score. DD-Net outperforms baseline CNN and SVM, and adding OF loss to baseline CNN produces a slight improvement in metrics. DeepRank (Method 11) had high confidence on predictions and poor performance on sparse classes, suggesting an overfitting problem that encourages the use of a simple ordinal loss for our small dataset.
Table 3:
Inter-rater Agreement Metrics.
| Rater 1 | Rater 2 | κ | Agr. (%) | F 1 | Pre | Rec |
|---|---|---|---|---|---|---|
| A | B | 0.38 | 47.3 | 0.51 | 0.64 | 0.49 |
| A | C | 0.39 | 56.4 | 0.59 | 0.73 | 0.58 |
| B | C | 0.30 | 56.4 | 0.62 | 0.72 | 0.68 |
|
| ||||||
| Average | 0.35 | 53.4 | 0.57 | 0.70 | 0.58 | |
Age Effects.
As seen in Table 1, the gait experiment involved participants with increasing average age as the MDS-UPDRS gait scores increased. To investigate the age effects on our estimation model (OF-DDNet with RCE), we conducted two statistical tests. The first test is an ANOVA (Analysis of Variance) (Anscombe, 1948) on all the participants in the group with ground-truth MDS-UPDRS score 1 (the largest group) and assesses whether their age is associated with the predicted score by our model. Fig. 8 shows the age distribution of these participants, some of which were incorrectly estimated as scores 0, 2, or 3. The ANOVA test on these 4 groups returned a p-value of 0.22, which indicates that there is no significant age differences across the groups. The second test is a general linear model regressing the predicted label from MDS-UPDRS with age being a covariate. The resulting p-value associated with age is 0.21. The insignificant results of these two tests verify that our model is not biased by age, even though the dataset was skewed with respect to age across the four MDS-UPDRS groups.
Figure 8:

Participants with ground-truth MDS-UPDRS scores of 1 plotted with their predicted score by our method and their age. The orange dots show the age of each participant. ANOVA test returned a p-value=0.22, confirming insignificant difference between the 4 groups concerning the participants’ age.
Model Interpretation.
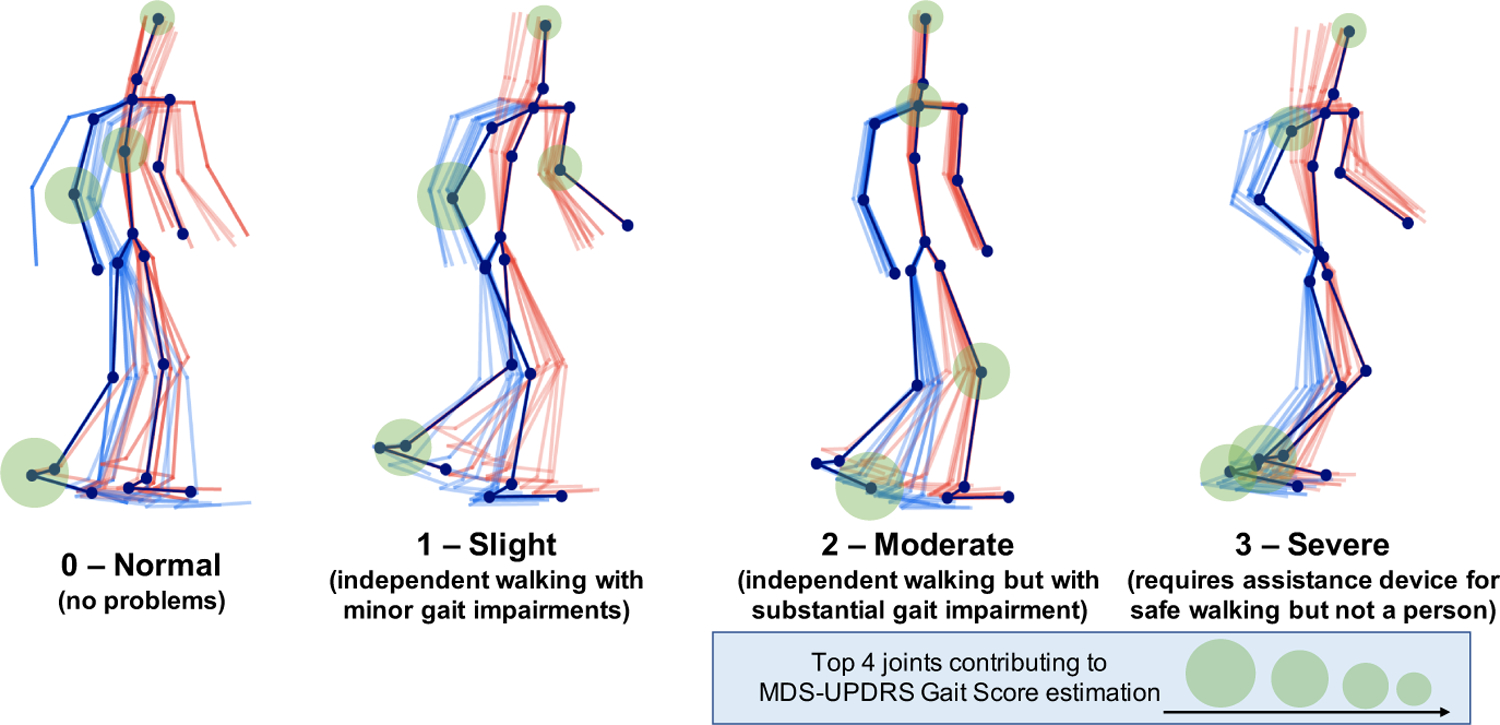
To understand which body joints contributed the most in correctly estimating the MDS-UPDRS gait scores, we provide saliency visualizations based on the size of the gradient updates to our model. Saliency is calculated as the average gradient update size per joint normalized to a scale from 0 to 1. Fig. 9 shows the saliency of 4 participants, whose gait trajectories are visualized in Fig. 1). Heels, ankles and toes have highest saliency overall, with moderate values around the arms and torso. Examiners are directed by the MDS-UPDRS rubric to pay attention to “stride amplitude, stride speed, height of foot lift, heel strike during walking, turning, and arm swing”. The position of the saliency matches the body parts that clinicians are instructed to pay attention to by the MDS-UPDRS exam, namely the feet (particularly heels) and arms. Our saliency analysis thus expresses significant promise in an interpretable fashion that our method is using the correct features to perform prediction. In the specific cases shown in Fig. 9, the participant with Score 0 has highest saliency at the right heel and right arm. In the Score 1 example, the participant’s feet have moderate saliency, particularly the left ankle. The arms and upper torso also represent moderate saliency, with very high saliency at the right elbow. This is corroborated by the video exam, as this patient has a stationary right arm with no bend. On the other hand, the left arm has a moderate arm swing, thus the high saliency in only the right arm matches clinical expectations. The participant with Score 2 has right toes and left knee with highest saliency. The initial clips did not have high saliency at the knee, but after the patient turned and started walking back, saliency at the left knee greatly intensified. In the score 3 sample, the model places very high saliency at both ankles. This participant’s ankles do not bend at all when walking, which is one of the primary indicators of severe gait impairment and should be scored as 3.
Figure 9:
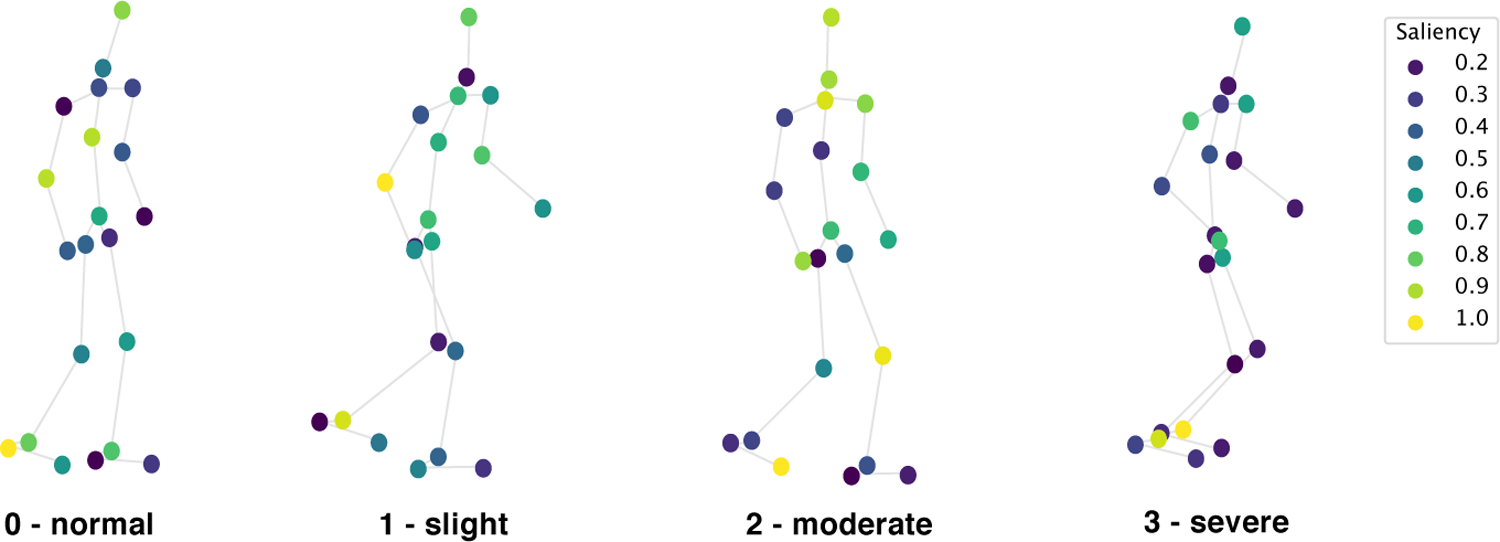
Saliency for the same participants as in Fig.1 visualized on the input joints, measured as normalized gradient update. Saliency is highest at the ankles, heels, and toes, with values sometimes high at the arms and knees.
We additionally produce and visualize the learned confusion matrices of the mean CM estimates for each rater in Fig. 10. Recall that the confusion matrix A(r) for rater r is the probability that r rates the ground-truth score yi = c as . This probability characterizes the systematic bias of rater r. All raters have strongest confidence on the diagonal, showing a clear pattern of partial consensus with our “ground-truth” learned by our model. Rater C has the highest incidence with ground-truth for score 0 and 2, Rater A has the highest such incidence for score 1, and Rater B for score 2. This shows our raters each having highest co-incidence with ground-truth for specific classes. Rater B shows a trend of “overestimating” the gait score for 1 and 2 compared to our ground-truth, while rater C tends to “underestimate.” Rater A also “underestimates” with highest confusion between classes 0 and 1. Furthermore, Score 1 consistently has the lowest accordance with the ground-truth in the confusion matrices for all three raters. Due to the nature of our ground-truth scores representing the majority vote, we note that these characterizations are not indicative of the quality of the scores, but rather their consensus with the majority vote among our clinicians. Thus, “over” and “under” estimation represent equally valid conservative opinions which either maximize for sensitivity or specificity.
Figure 10:

Visualization of the confusion matrices of the learned CM estimates for each rater (Raters A, B, C) averaged over all folds.
4.2. Finger Tapping Score Estimation Results - Single Rater Scores
For this experiment, only one rater scores the videos, so we run our OF-DDNet without RCE. The results of our finger tapping score estimation and baselines are listed in Table 4. Our method, OF-DDNet, achieves a macro-average AUC of 0.69, F1-score of 0.47, precision of 0.47, and balanced accuracy (average recall) of 48%. We compare this to three baselines, Baseline DD-Net (Yang et al., 2019) without OF loss, Baseline Temporal CNN (denoted by TCNN, 1D CNN on the joint 3D coordinates in time), and a support vector machine (SVM) with fixed size input clips of 100 frames each (Weston et al., 1999). OF-DDNet significantly outperforms the baselines by the Wilcoxon signed rank test (Wilcoxon, 1992) (p < 0.05) performed on video clips. Both DD-Net-based methods achieve higher metrics than TCNN and SVM. The per-class finger tapping score predictions of our method, visualized in Fig. 11, show that MDS-UPDRS scores 1 to 3 have fairly balanced metrics, while score 0 has high AUC but low recall, which is caused by the small number of samples with score 0 (N = 5). Nevertheless, the balanced accuracy, which takes into account the imbalanced number of samples across scores, is significantly better than chance (null classifier) based on permutation testing (one-tailed p = 0.003).
Table 4:
Comparison of MDS-UPDRS finger tapping score estimation.
| Method | F 1 | AUC | Pre | bAcc |
|---|---|---|---|---|
| Ours (OF-DDNet) | 0.47 | 0.69 | 0.47 | 0.48 |
| DD-Net* (Yang et al., 2019) | 0.41 | 0.68 | 0.41 | 0.41 |
| TCNN* | 0.39 | 0.64 | 0.39 | 0.40 |
| SVM* | 0.35 | 0.59 | 0.35 | 0.36 |
indicates statistical difference at (p < 0.05) compared with our method, measured by the Wilcoxon signed rank test (Wilcoxon, 1992). Best results are in bold.
Figure 11:
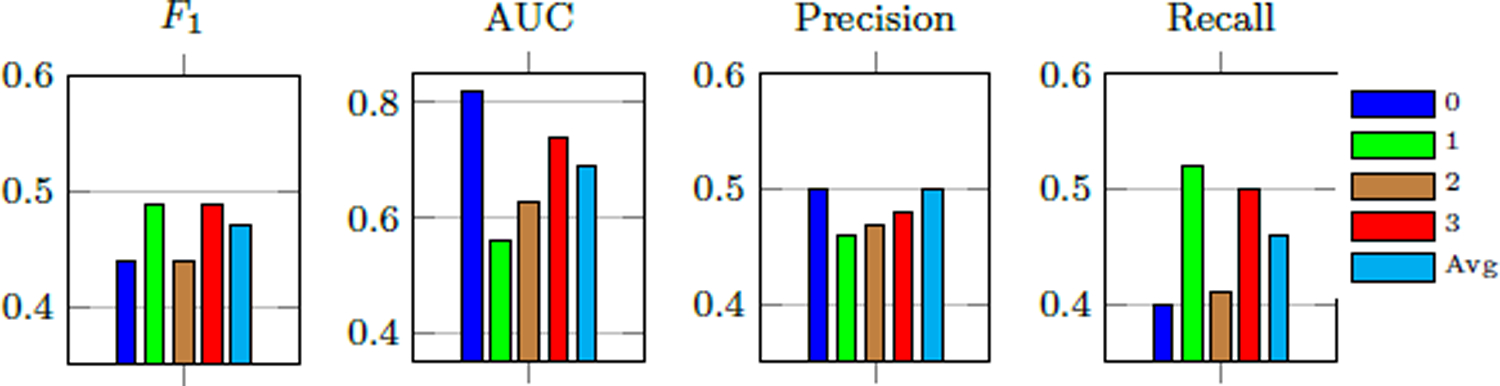
Per-class MDS-UPDRS finger tapping score estimation performance of our method.
Model Interpretation.
We similarly present saliency visualizations for the finger tapping test in Fig. 12. Saliency is measured as normalized gradient update on the input joints of four subjects with different scores. Saliency is highest at the thumb and index finger for all classes, which aligns with what the MDS-UPDRS rubric instructs examiners to observe, namely the speed, amplitude, hesitations, halts, and decrementing amplitude of the tapping between thumb and index finger.
Figure 12:
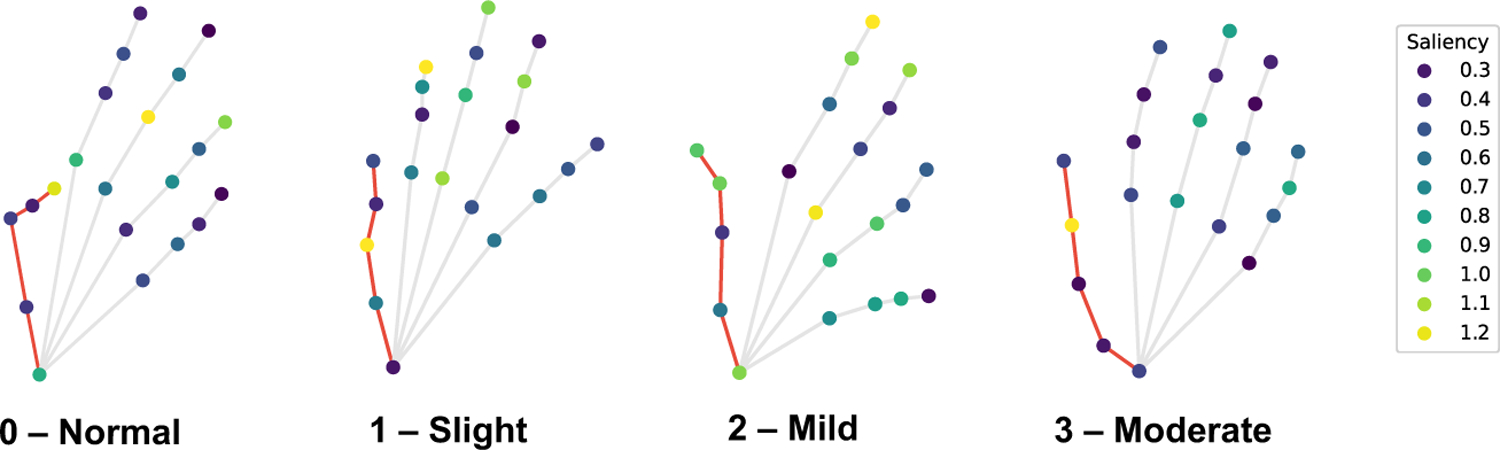
Saliency measured as normalized gradient update for the finger-tapping test on the input joints of four subjects with MDS-UPDRS finger tapping score shown. Thumb joints are connected with red edges. Saliency is highest at the thumb and index finger.
5. Discussion
In this work, we demonstrated a method to predict MDS-UPDRS gait scores despite multiple noisy scores and the possibility of extension to other types of PD motor severity evaluation, such as the finger tapping test. Despite high noise from inter-rater scores (see Table 3), our method achieved compelling results on the gait model by utilizing the full distribution of ratings for a dataset with multiple scores. As this is both a highly challenging and common problem in machine learning, computer vision, and particularly medical applications, demonstrating the high efficacy of this solution could be helpful in modeling uncertainty in clinical ratings.
There is inherent subjectivity in the MDS-UPDRS scale (Evers et al., 2019) despite attempts to standardize the exam through objective criterion (e.g., stride amplitude/speed, heel strike, arm swing). Physicians often disagree on ambiguous cases and lean toward one score versus another based on subtle cues. Our results are consistent with the markers that examiners are directed by the MDS-UPDRS rubric to pay attention to, namely the feet (particularly heels) and arms in the gait test and the index finger and thumb of the finger tapping test. Our saliency analysis makes our results more interpretable and shows that our method is using the correct features to perform prediction. As corroborated in the results of our method, OF-DDNet with RCE, the most difficult classes to categorize in clinical practice are scores 1 and 2 since the MDS-UPDRS defines its distinction from score 1 solely by “minor” versus “substantial” gait impairment, shown in Fig. 1. This was also verified by a test-retest reliability analysis between A* and A (scoring and rescoring of the rater A of those participants that we have scores for both ratings), which resulted in a Pearson correlation of r = 0.69 and an intraclass correlation of ICC = 0.60. The test-retest yielded (κ = 0.38,F1 = 0.4,precision = 0.52,recall = 0.50). Taking either set of ratings to determine the “true” class (represented as either precision or recall) produces bAcc around only 50.0%. In comparison, our method strongly classified the data and is superior according to these metrics against the majority vote, showing the reliability of our estimation (Table 2).
Scoring the motor MDS-UPDRS exam is known to have high inter-observer variability, which we empirically evaluated to discover average Cohen’s Kappa coefficient 0.35 and an agreement of 53.4%. Our method achieved a Cohen’s kappa of 0.49 in comparison, much higher than between all rater pairs. Crucially, this demonstrates our method’s ability to synthesize score distributions and rater confusion estimation and generalize past the noise to find the salient patterns shared by all raters.
Our saliency visualizations provided further evidence that our model is using valid salient features from the input keypoints to make predictions. These predictions often match the features that clinicians use to rate participants, such as a concentration of attention in the heels, ankles, and toe areas of the feet, as well as conditional consideration of arm swing, bend in the knees, and overall stiffness in the torso. For different classes and participants, our model was able to identify unique salient features for each class and for unique participants
We see from Table 2 that performance improves with increased information from multiple raters. Combining all scores into a majority vote during training omits information about the distribution of ratings and thereby resulted in the lowest performance. When multiple rater information was more wholly retained in the form of soft scores (Table 2, methods 4 and 5), this improved results over the aggregated labels. Further preserving multiple rater data by modeling rater confusion as explained in Sec. 3.2.3 with dual training of the estimated score distribution and the rater confusion matrices (via RCE) produced our highest performing model. In comparison, when trained on scores from a single rater, as in our previous work (Lu et al., 2020), ratings may be systematically biased due to rating habits or subjective opinion of a single rater. This may enable a model to learn the habits of a single rater very well, but risks generalizability when compared with the ‘true score’ across multiple raters. The level of inter-observer variability depends significantly on factors such as the application, observer expertise, and attention (Lampert et al., 2016). Our results suggest that when scores from multiple experts are available, methods that model observer confusion as part of the training process generally perform better than methods that aggregate the scores in a separate step prior to training. Our results also showed significant gains from using scores provided by multiple experts. This is demonstrated by the higher performance of the Rater Confusion Estimation and Soft Label models (1, 2, 3, 4, 5, 6) over the pre-aggregated Majority Vote models (6, 7) (see Table 2). There is opportunity for further exploration of other loss configurations, such as KL divergence, which exhibited comparable performance to softmax. In this work, we chose CE for simplicity due to known formulations with the losses we used (e.g., focal loss) and easier side-by-side comparison to other methods.
Furthermore, as in (Lu et al., 2020), we again show the effectiveness of our redefined hybrid ordinal-focal loss on the additional finger tapping experiment for tempering the effects of a small, imbalanced dataset and leveraging the ordinal nature of the MDS-UPDRS. A score of 0 is especially difficult for our model to classify, which corroborates clinical rating in which score 0 may be frequently labeled as score 1 if there are subtle but visible disturbances. The finger tapping test demonstrates the extensibility of our method to other aspects of the MDS-UPDRS exam besides the gait test.
This study addresses several limitations in our previous work (Lu et al., 2020). Our previous dataset had a shortage of score 0 participants in our clinical dataset, so we included examples of non-PD gait from the public CASIA dataset. The data was obfuscated by converting to normalized skeletons, which has similar characteristics across both datasets, so should theoretically adequately represent score 0. However, we mentioned that expanding the clinical dataset by recruiting more participants from underrepresented classes would strengthen the results by presenting a more homogeneous data collection across classes. In this work, we thus collected 25 new videos with 9 additional score 0 exams. Furthermore, by nearly doubling our dataset, we have partially remedied another primary concern of our previous work, the relatively small dataset size carrying risk of overfitting in the results.
This study presented a few additional limitations. Firstly, our datasets are still relatively small, which carries risk of overfitting and uncertainty in the results. We mitigated this through data augmentation techniques and using simple models (DD-Net) instead of deep or complex network architectures; and the latter with leave-one-out cross validation instead of the traditional train/validation/test split used in deep learning community. Similarly, our dataset is still imbalanced with considerably fewer examples with score 3 of the gait experiment and scores 0 and 1 of the finger tapping experiment, which we attempted to address through our custom ordinal focal loss.
The primary concern of this work is the lack of objective “ground-truth” scores, which do not exist and are possibly unable to be validated. We generate such a pseudo ground-truth label set by taking the majority vote of the raters, but this technique carries some caveats. Furthermore, our score 0 cohort has noticeably younger average age than the rest of the classes, which could be serving as a confounder. However, we showed through ANOVA testing that our model learned the MDS-UPDRS score estimation unbiased to age differences. Collecting data from more score 0 older participants can mitigate this risk, although it may be challenging as older adults often have at least minor impairments in their gait performance.
6. Conclusion
Herein, we proposed a method for robust estimation of PD motor severity from two different MDS-UPDRS exams (gait and finger-tapping tests) and across noisy scores from multiple raters. This extended our previous work (Lu et al., 2020), which presented the first benchmark to assess PD severity from only gait videos, by incorporating multiple raters for the gait experiment and adding an additional study on the finger tapping exam. Since MDS-UPDRS gait scores contain high rating noise and uncertainty, our method addressed the case of multiple raters with sizable disagreement, a crucial roadblock to clinical deployment and transfer to real-world settings. In addition, we demonstrated that our method is extensible to other MDS-UPDRS tests besides gait via the new finger tapping experiment. Our proposed techniques for handling model uncertainty or label noise with RCE and addressing small dataset and imbalance issues with OF-DDNET have the opportunity for application to similar video classification problems in clinical applications.
Highlights.
We propose a generic pipeline for estimating movement impairment severity scores using body or hand skeletons and classify them into clinical scores.
We assess the inter-rater reliability of multiple ratings from 3 different raters and propose a pipeline to learn clinical score estimation under uncertainty.
We extend our model via the Rater Confusion Estimation framework trained by our novel ordinal focal loss, with the addition of an explicit simplex projection for learning.
We present saliency visualizations to stratify the contribution of separate body joints to the estimation of MDS-UPDRS scores.
7. Acknowledgments
This research was supported in part by NIH grants AA010723, AA017347, AG047366, and P30AG066515. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also supported by the Stanford School of Medicine Department of Psychiatry & Behavioral Sciences 2021 Innovator Grant Program and the Stanford Institute for Human-centered Artificial Intelligence (HAI) AWS Cloud Credit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has conflicts of interest with the reported data or their interpretation.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this papers
References
- Adeli E, Shi F, An L, Wee CY, Wu G, Wang T, Shen D, 2016. Joint feature-sample selection and robust diagnosis of parkinson’s disease from mri data. NeuroImage 141, 206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeli V, Adeli E, Reid I, Niebles JC, Rezatofighi H, 2020. Socially and contextually aware human motion and pose forecasting. IEEE Robotics and Automation Letters 5, 6033–6040. [Google Scholar]
- Andriluka M, Pishchulin L, Gehler P, Schiele B, 2014. 2d human pose estimation: New benchmark and state of the art analysis, in: Proceedings of the IEEE Conference on computer Vision and Pattern Recognition, pp. 3686–3693. [Google Scholar]
- Anscombe FJ, 1948. The validity of comparative experiments. Journal of the royal statistical society. series A (General) 111, 181–211. [PubMed] [Google Scholar]
- Arteta C, Lempitsky V, Zisserman A, 2016. Counting in the wild, in: European conference on computer vision, Springer. pp. 483–498. [Google Scholar]
- Babu AR, Zakizadeh M, Brady JR, Calderon D, Makedon F, 2019. An intelligent action recognition system to assess cognitive behavior for executive function disorder, in: 2019 IEEE 15th International Conference on Automation Science and Engineering (CASE), IEEE. pp. 164–169. [Google Scholar]
- Benatru I, Vaugoyeau M, Azulay JP, 2008. Postural disorders in parkinson’s disease. Neurophysiologie Clinique/Clinical Neurophysiology 38, 459–465. [DOI] [PubMed] [Google Scholar]
- Bharti K, Suppa A, Tommasin S, Zampogna A, Pietracupa S, Berardelli A, Pantano P, 2019. Neuroimaging advances in parkinson’s disease with freezing of gait: A systematic review. NeuroImage: Clinical, 102059. [DOI] [PMC free article] [PubMed]
- Cabral RS, Torre F, Costeira JP, Bernardino A, 2011. Matrix completion for multi-label image classification, in: Advances in neural information processing systems, Citeseer. pp. 190–198. [Google Scholar]
- Chandra SR, Issac TG, Abbas MM, 2015. Apraxias in neurodegenerative dementias. Indian journal of psychological medicine 37, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H.k., Adeli E, Wang B, Huang DA, Niebles JC, 2019. Action-agnostic human pose forecasting, in: 2019 IEEE Winter Conference on Applications of Computer Vision (WACV), IEEE. pp. 1423–1432. [Google Scholar]
- Cho CW, Chao WH, Lin SH, Chen YY, 2009. A vision-based analysis system for gait recognition in patients with parkinson’s disease. Expert Systems with applications 36, 7033–7039. [Google Scholar]
- Clarke C, Patel S, Ives N, Rick C, Woolley R, Wheatley K, et al. , 2016. Uk parkinson’s disease society brain bank diagnostic criteria. NIHR Journals Library. [Google Scholar]
- Criss K, McNames J, 2011. Video assessment of finger tapping for parkinson’s disease and other movement disorders, in: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE. pp. 7123–7126. [DOI] [PubMed] [Google Scholar]
- Daneault JF, Vergara-Diaz G, Parisi F, Admati C, Alfonso C, Bertoli M, Bonizzoni E, Carvalho GF, Costante G, Fabara EE, et al. , 2021. Accelerometer data collected with a minimum set of wearable sensors from subjects with parkinson’s disease. Scientific Data 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dgani Y, Greenspan H, Goldberger J, 2018. Training a neural network based on unreliable human annotation of medical images, in: 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), IEEE. pp. 39–42. [Google Scholar]
- Ellis T, Cavanaugh JT, Earhart GM, Ford MP, Foreman KB, Dibble LE, 2011. Which measures of physical function and motor impairment best predict quality of life in parkinson’s disease? Parkinsonism & related disorders 17, 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers LJ, Krijthe JH, Meinders MJ, Bloem BR, Heskes TM, 2019. Measuring parkinson’s disease over time: The real-world within-subject reliability of the mds-updrs. Movement Disorders 34, 1480–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Pongmala C, Artusi CA, Imbalzano G, Romagnolo A, Lopiano L, Zibetti M, 2020. Video analysis of long-term effects of levodopa-carbidopa intestinal gel on gait and posture in advanced parkinson’s disease. Neurological Sciences , 1–4. [DOI] [PubMed]
- Gattupalli S, Ebert D, Papakostas M, Makedon F, Athitsos V, 2017. Cognilearn: A deep learning-based interface for cognitive behavior assessment, in: Proceedings of the 22nd International Conference on Intelligent User Interfaces, pp. 577–587. [Google Scholar]
- Ghosh A, Kumar H, Sastry P, 2017. Robust loss functions under label noise for deep neural networks, in: Proceedings of the AAAI Conference on Artificial Intelligence. [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, et al. , 2008. Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (mds-updrs): scale presentation and clinimetric testing results. Movement disorders: official journal of the Movement Disorder Society 23, 2129–2170. [DOI] [PubMed] [Google Scholar]
- Gulli A, Pal S, 2017. Deep learning with Keras. Packt Publishing Ltd. [Google Scholar]
- Han J, Jeon HS, Jeon BS, Park KS, 2006. Gait detection from three dimensional acceleration signals of ankles for the patients with parkinson’s disease, in: Proceedings of the IEEE The International Special Topic Conference on Information Technology in Biomedicine, Ioannina, Epirus, Greece. [Google Scholar]
- Heilman KM, Rothi LJ, Valenstein E, 1982. Two forms of ideomotor apraxia. Neurology 32, 342–342. [DOI] [PubMed] [Google Scholar]
- Hobert MA, Nussbaum S, Heger T, Berg D, Maetzler W, Heinzel S, 2019. Progressive gait deficits in parkinson’s disease: A wearable-based biannual 5-year prospective study. Frontiers in aging neuroscience 11, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hssayeni MD, Jimenez-Shahed J, Burack MA, Ghoraani B, 2019. Wearable sensors for estimation of parkinsonian tremor severity during free body movements. Sensors 19, 4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu C, Papava D, Olaru V, Sminchisescu C, 2013. Human3. 6m: Large scale datasets and predictive methods for 3d human sensing in natural environments. IEEE transactions on pattern analysis and machine intelligence 36, 1325–1339. [DOI] [PubMed] [Google Scholar]
- Izadinia H, Russell BC, Farhadi A, Hoffman MD, Hertzmann A, 2015. Deep classifiers from image tags in the wild, in: Proceedings of the 2015 Workshop on Community-Organized Multimodal Mining: Opportunities for Novel Solutions, pp. 13–18. [Google Scholar]
- Kanazawa A, Black MJ, Jacobs DW, Malik J, 2018. End-to-end recovery of human shape and pose, in: CVPR, pp. 7122–7131. [Google Scholar]
- Karimi D, Dou H, Warfield SK, Gholipour A, 2020. Deep learning with noisy labels: Exploring techniques and remedies in medical image analysis. Medical Image Analysis 65, 101759. URL: https://www.sciencedirect.com/science/article/pii/S1361841520301237, doi: 10.1016/j.media.2020.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma DP, Ba J, 2014. Adam: A method for stochastic optimization. arXiv preprint arXiv:1412.6980.
- Kocabas M, Athanasiou N, Black MJ, 2020. Vibe: Video inference for human body pose and shape estimation, in: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, pp. 5253–5263. [Google Scholar]
- Kwon H, Abowd GD, Plötz T, 2019. Handling annotation uncertainty in human activity recognition, in: Proceedings of the 23rd International Symposium on Wearable Computers, pp. 109–117. [Google Scholar]
- Lampert TA, Stumpf A, Gançarski P, 2016. An empirical study into annotator agreement, ground truth estimation, and algorithm evaluation. IEEE Transactions on Image Processing 25, 2557–2572. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM, 1998. Parkinson’s disease. New England Journal of Medicine 339, 1130–1143. [DOI] [PubMed] [Google Scholar]
- LeCun Y, Bengio Y, Hinton G, 2015. Deep learning. nature 521, 436–444. [DOI] [PubMed] [Google Scholar]
- Lee CY, Kang SJ, Hong SK, Ma HI, Lee U, Kim YJ, 2016. A validation study of a smartphone-based finger tapping application for quantitative assessment of bradykinesia in parkinson’s disease. PloS one 11, e0158852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shao X, Zhang C, Qian X, 2021. Automated assessment of parkinsonian finger-tapping tests through a vision-based fine-grained classification model. Neurocomputing 441, 260–271. [Google Scholar]
- Lin B, Luo W, Luo Z, Wang B, Deng S, Yin J, Zhou M, 2020. Bradykinesia recognition in parkinson’s disease via single rgb video. ACM Transactions on Knowledge Discovery from Data (TKDD) 14, 1–19. [Google Scholar]
- Lin TY, Goyal P, Girshick R, He K, Dollár P, 2017. Focal loss for dense object detection, in: CVPR, pp. 2980–2988. [DOI] [PubMed] [Google Scholar]
- Long C, Hua G, 2015. Multi-class multi-annotator active learning with robust gaussian process for visual recognition, in: Proceedings of the IEEE international conference on computer vision, pp. 2839–2847. [Google Scholar]
- Loper M, Mahmood N, Romero J, Pons-Moll G, Black MJ, 2015. Smpl: A skinned multi-person linear model. ACM Trans on graphics 34, 1–16. [Google Scholar]
- Lu M, Poston K, Pfefferbaum A, Sullivan EV, Fei-Fei L, Pohl KM, Niebles JC, Adeli E, 2020. Vision-based estimation of MDS-UPDRS gait scores for assessing Parkinson’s disease motor severity, in: International Conference on Medical Image Computing and Computer-Assisted Intervention, Springer. pp. 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q, Qin W.z., Zhang A, Ye N, 2020. Recent advances in dopaminergic strategies for the treatment of parkinson’s disease. Acta Pharmacologica Sinica 41, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcante A, Di Marco R, Gentile G, Pellicano C, Assogna F, Pontieri FE, Spalletta G, Macchiusi L, Gatsios D, Giannakis A, et al. , 2021. Foot pressure wearable sensors for freezing of gait detection in parkinson’s disease. Sensors 21, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Martín P, Rodríguez-Blázquez C, Alvarez M, Arakaki T, Arillo VC, Chaná P, Fernández W, Garretto N, Martínez-Castrillo JC, Rodríguez-Violante M, et al. , 2015. Parkinson’s disease severity levels and mds-unified parkinson’s disease rating scale. Parkinsonism & related disorders 21, 50–54. [DOI] [PubMed] [Google Scholar]
- McHugh ML, 2012. Interrater reliability: the kappa statistic. Biochemia medica 22, 276–282. [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Sridhar S, Sotnychenko O, Rhodin H, Shafiei M, Seidel HP, Xu W, Casas D, Theobalt C, 2017. Vnect: Real-time 3d human pose estimation with a single rgb camera. URL: http://gvv.mpi-inf.mpg.de/projects/VNect/, doi: 10.1145/3072959.3073596. [DOI]
- Nair T, Precup D, Arnold DL, Arbel T, 2020. Exploring uncertainty measures in deep networks for multiple sclerosis lesion detection and segmentation. Medical image analysis 59, 101557. [DOI] [PubMed] [Google Scholar]
- Napier TC, Kirby A, Persons AL, 2020. The role of dopamine pharmacotherapy and addiction-like behaviors in parkinson’s disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry 102, 109942. [DOI] [PubMed] [Google Scholar]
- Negin F, Rodriguez P, Koperski M, Kerboua A, Gonzàlez J, Bourgeois J, Chapoulie E, Robert P, Bremond F, 2018. Praxis: Towards automatic cognitive assessment using gesture recognition. Expert systems with applications 106, 21–35. [Google Scholar]
- Nguyen Q, Valizadegan H, Hauskrecht M, 2014. Learning classification models with soft-label information. Journal of the American Medical Informatics Association 21, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Lan Y, Guo J, Xu J, Xu J, Cheng X, 2017. Deeprank: A new deep architecture for relevance ranking in information retrieval, in: Proceedings of the 2017 ACM on Conference on Information and Knowledge Management, pp. 257–266. [Google Scholar]
- Pham HH, Le TT, Tran DQ, Ngo DT, Nguyen HQ, 2019. Interpreting chest x-rays via cnns that exploit disease dependencies and uncertainty labels. medRxiv , 19013342.
- Poston KL, YorkWilliams S, Zhang K, Cai W, Everling D, Tayim FM, Llanes S, Menon V, 2016. Compensatory neural mechanisms in cognitively unimpaired p arkinson disease. Annals of neurology 79, 448–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo A, Mehdizadeh S, Ng KD, Iaboni A, Taati B, 2020. Assessment of parkinsonian gait in older adults with dementia via human pose tracking in video data. Journal of neuroengineering and rehabilitation 17, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Joo H, Matthews I, Sheikh Y, 2017. Hand keypoint detection in single images using multiview bootstrapping, in: CVPR. [Google Scholar]
- Sivaranjini S, Sujatha C, 2020. Deep learning based diagnosis of parkinson’s disease using convolutional neural network. Multimedia Tools and Applications 79, 15467–15479. [Google Scholar]
- Stamatakis J, Ambroise J, Crémers J, Sharei H, Delvaux V, Macq B, Garraux G, 2013. Finger tapping clinimetric score prediction in parkinson’s disease using low-cost accelerometers. Computational intelligence and neuroscience 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker M, Hinde D, Rolland A, Salzman N, Watson A, Almonroeder TG, 2021. Quantifying step length using two-dimensional video in individuals with parkinson’s disease. Physiotherapy theory and practice 37, 252–255. [DOI] [PubMed] [Google Scholar]
- Tanno R, Saeedi A, Sankaranarayanan S, Alexander DC, Silberman N, 2019. Learning from noisy labels by regularized estimation of annotator confusion, in: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, pp. 11244–11253. [Google Scholar]
- Venuto CS, Potter NB, Ray Dorsey E, Kieburtz K, 2016. A review of disease progression models of parkinson’s disease and applications in clinical trials. Movement Disorders 31, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Peng X, Yang J, Lu S, Qiao Y, 2020. Suppressing uncertainties for large-scale facial expression recognition, in: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, pp. 6897–6906. [Google Scholar]
- Wang X, Kodirov E, Hua Y, Robertson NM, 2019. Improving mae against cce under label noise. arXiv preprint arXiv:1903.12141.
- Weston J, Watkins C, et al. , 1999. Support vector machines for multi-class pattern recognition., in: Esann, pp. 219–224. [Google Scholar]
- Wilcoxon F, 1992. Individual comparisons by ranking methods, in: Break-throughs in statistics. Springer, pp. 196–202. [Google Scholar]
- Xue D, Sayana A, Darke E, Shen K, Hsieh JT, Luo Z, Li LJ, Downing NL, Milstein A, Fei-Fei L, 2018. Vision-based gait analysis for senior care. arXiv preprint arXiv:1812.00169.
- Yang F, Wu Y, Sakti S, Nakamura S, 2019. Make skeleton-based action recognition model smaller, faster and better, in: ACM Multimedia Asia, pp. 1–6. [Google Scholar]


