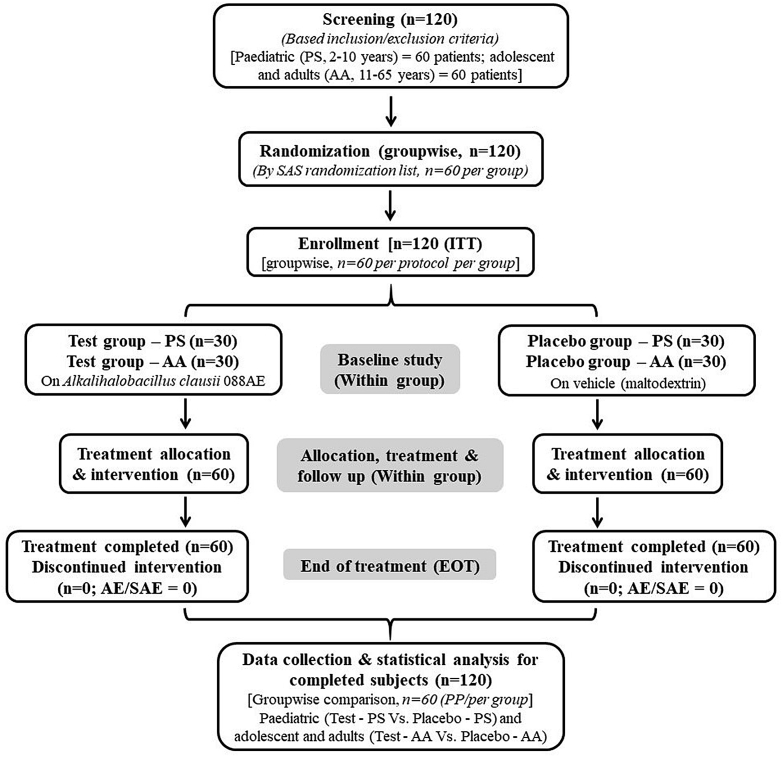
Figure 1.
Schematic diagram of the clinical study on evaluation of the efficacy and safety of Alkalihalobacillus clausii 088AE in the treatment of antibiotic-associated diarrhea. The study enrolled total 120 intension to treat (ITT) subjects (each 60 subjects in pediatric group and adolescent and adult group) through the inclusion and exclusion criteria screening. Each age group was allocated with 60 subjects (each 30 subjects in test and placebo arm) following the SAS random number generation method. With no adverse effects (AEs) or serious adverse events (SAEs) report and no discontinuation of subjects, completed 120 per protocol (PP) subjects were considered for endpoints evaluation. Data comparison between test and placebo, and statistical analysis were performed within age group. Study compliance was checked at every follow up visits by a team of physician-investigator and officers.