ABSTRACT
Background
Cerebrospinal fluid (CSF) levels of monoamine metabolites may represent biomarkers of Parkinson's disease (PD).
Objective
The aim of this study was quantification of multiple metabolites in CSF from PD versus healthy control subjects (HCs), including longitudinal analysis.
Methods
Absolute levels of multiple monoamine metabolites in CSF were quantified by liquid chromatography coupled with tandem mass spectrometry from 161 individuals with early PD and 115 HCs from the Parkinson's Progression Marker Initiative and de novo PD (DeNoPA) studies.
Results
Baseline levels of homovanillic acid (HVA) and 3,4‐dihydroxyphenylacetic acid (DOPAC) were lower in individuals with PD compared with HCs. HVA levels correlated with Movement Disorder Society Unified Parkinson's Disease Rating Scale total scores (P < 0.01). Both HVA/dopamine and DOPAC/dopamine levels correlated with caudate nucleus and raw DOPAC with putamen dopamine transporter single‐photon emission computed tomography uptake ratios (P < 0.01). No metabolite changed over 2 years in drug‐naive individuals, but some changed on starting levodopa treatment.
Conclusions
HVA and DOPAC CSF levels mirrored nigrostriatal pathway damage, confirming the central role of dopaminergic degeneration in early PD. © 2021 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: monoamine metabolites, catecholamine, neurotransmitter, biomarker, Parkinson's disease, CSF, homovanillic acid
Parkinson's disease (PD) is characterized by progressive loss of dopaminergic neurons in the substantia nigra pars compacta,1 but also depletion of other neurotransmitters, such as serotonin in the striatum and noradrenaline in the hypothalamus and frontal cortex.2, 3 Cerebrospinal fluid (CSF) represents the most proximal source of molecular biomarkers for these deficiencies.4 Although quantification of CSF protein biomarkers improves early diagnosis in Alzheimer's disease,5 no analogous protein biomarkers for PD diagnosis exist. Neurotransmitter metabolites represent a potential proxy to PD‐specific neurodegeneration and may serve as promising biomarkers of disease severity and its progression.
Several studies investigating dopamine metabolites in PD found consistent signatures, in particular, decreased levels of the main dopamine metabolite homovanillic acid (HVA).6, 7, 8, 9, 10 However, their utility for monitoring disease progression has been questioned, mainly because of the results of the DATATOP (deprenyl and tocopherol antioxidative therapy of parkinsonism) study in which repeated CSF measurements of dopamine metabolites by gas chromatography–mass spectrometry yielded variable results. Despite efforts to standardize CSF collection, processing, and measurement,11, 12 potential confounding factors on catecholamine levels remain (eg, diurnal changes, total CSF volume) and may impede the reliable quantification.13, 14, 15, 16
Although high‐performance liquid chromatography with electrochemical detection (HPLC‐ECD) and gas chromatography–mass spectrometry were previously considered gold standards for analyzing dopamine and its metabolites,7, 11 LC–MS/MS (liquid chromatography coupled with tandem mass spectrometry) has evolved during the last two decades with comparable sensitivity to HPLC‐ECD and greatly improved selectivity.17, 18 LC–MS/MS reduces the complexity of preanalytical processing17 and is now considered the gold standard for quantitative analytics. This enables simultaneous analyses of metabolites of the dopaminergic (eg, 3,4‐dihydroxyphenylalanine [DOPA], dopamine, 3,4‐dihydroxyphenylacetic acid [DOPAC]), noradrenergic (eg, 3,4‐dihydroxyphenylglycol, 4‐hydroxy‐3‐methoxyphenylglycol) and serotonergic (eg, 5‐hydroxy‐3‐indoleacetic acid [5‐HIAA]) pathways in biofluids, including CSF.19
We for the first time applied LC–MS/MS to measure multiple monoamine metabolite concentrations in human CSF samples from the single‐center de novo PD (DeNoPa)‐cohort,20, 21 including longitudinal analysis in the multicenter Parkinson's Progressive Markers Initiative (PPMI)22, 23, 24 study, to assess their utility as biomarkers of both PD severity and its progression.
Materials and Methods
Study Participants and CSF Sampling Procedure
DeNoPa Cohort
CSF baseline samples from 49 age‐ and sex‐matched healthy control subjects (HC) and 62 drug‐naive PD participants were analyzed from the DeNoPa study.20 CSF samples were collected and processed as previously described.25
PPMI Cohort
Baseline and 1‐year CSF samples from 56 HCs and 95 age‐, sex‐, body mass index (BMI)‐, and total CSF volume‐matched participants with dopamine transporter single‐photon emission computed tomography (DaT‐SPECT)‐confirmed PD were analyzed (https://www.ppmi-info.org/study-design/). Fifty‐four individuals with PD remained unmedicated at the 1‐year visit, while 39 individuals with PD had started l‐dopa medication. Two‐year follow‐up CSF samples were available from all 56 HCs and 39 individuals with PD, all of whom were on l‐dopa medication. Clinical and medication data were retrieved from the PPMI data portal (https://www.ppmi-info.org/access-data-specimens/download-data/). CSF samples were collected and processed following standardized procedures (https://www.ppmi-info.org/study-design/) (see also The Parkinson's Progression Marker Initiative24 and Kang26).
A comparison of CSF sampling procedures for DeNoPa and PPMI is provided in Table S4. Demographics and clinical characteristics for DeNoPa and PPMI are provided in Table S1. Both studies were approved by the ethics committees: in Frankfurt (Hessen, Germany) for DeNoPa and the Institutional Review Board of all participating sites for PPMI. Written informed consent was obtained from all participants before inclusion in the study.
Metabolite Quantification
Absolute metabolite quantification was performed at Metanomics Health GmbH, Germany. CSF samples were subjected to ultracentrifugation and dansyl chloride derivatization prior to solid‐phase extraction and LC–MS/MS analysis: data were normalized against internal standards and quantified using calibration standards as previously described.17, 27 The metabolite panels that were analyzed, including their limit of detection, are provided in Table S2. Technical robustness of the analytical method was confirmed in a subset of seven CSF randomly selected blinded samples from the DeNoPa study (see Table S3). Ratios were derived for analyses of metabolite levels normalized by the concentration of the respective neurotransmitter. Stringent procedures to minimize time between thawing and monoamine metabolite analysis were consistently applied for all samples.
Statistical Analysis
All statistical analyses were performed using R and are described in detail in the Supporting Data.
Results
Demographic and Clinical Data in the DeNoPa and PPMI Cohorts
Groups did not differ with respect to mean age (± standard deviation) (HC: 65.6 ± 6.6, PD: 64.1 ± 9.4, F = 1.2, P = 0.28) or sex distribution (HCs [male/female]: 30/19, PD: 42/20, χ2 = 0.51, P = 0.47) in the DeNoPa study. Groups differed with respect to Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS UPDRS) Part III (HC: 0.35 ± 1.03, PD: 19.4 ± 9.9, F = 386, P < 0.001) and total scores (HC: 3.1 ± 2.8, PD: 29.8 ± 15.6, F = 290, P < 0.001).
Groups had comparable baseline ages (mean ± standard deviation) (HC: 62.7 ± 10.7, PD: 62.4 ± 9.8, F = 0.21, P = 0.65), sex distributions (HCs [male/female]: 40/16, PD: 64/31, χ2 = 0.27, P = 0.6), BMI (HC: 26.8 ± 5.2, PD: 26.7 ± 4.2, F = 0.06, P = 0.95), and total CSF volume (HC: 17.8 ± 3.1, PD: 16.9 ± 2.9, F = 1.9, P = 0.15) but differed with respect to MDS UPDRS Part III (HC: 1.2 ± 2.0, PD: 21.1 ± 8.5, F = 346, P < 0.001) and total scores (HC: 4.7 ± 4.0, PD: 33.4 ± 13.5, F = 318, P < 0.001) in the PPMI study (see Table S1).
Quantifiable Metabolites
Overall, 8 of 17 metabolites could be quantified in the DeNoPa and 12 of 17 in the PPMI samples, and they were considered in further analyses. The upper levels of detection were reached in some PPMI PD samples for 3‐methoxytyrosine (25%) and DOPA (13%).
CSF Monoamine Metabolite Levels at Baseline
Given that dopamine levels in the DeNoPa cohort were mostly below the limit of detection for multiple samples, only nonratio metabolite and neurotransmitter levels were analyzed. Four metabolites differed between DeNoPa PD and HC groups: HVA (estimate = −0.41 ± 0.10, P < 0.0001, effect size = 0.13), 5‐HIAA (estimate = −0.32 ± 0.10, P = 0.002, effect size = 0.08), 4‐hydroxy‐3‐methoxyphenylglycol (estimate = −0.12 ± 0.04, P = 0.008, effect size = 0.05), and DOPAC (estimate = −0.25 ± 0.09, P = 0.06, effect size = 0.04) (see Table S5).
The DeNoPa findings were partially confirmed in the PPMI samples, in which HVA and DOPAC raw and normalized levels differed between HC and PD groups (HVA: estimate = −0.33 ± 0.07, P < 0.0001, effect size = 0.15; DOPAC: estimate = 0.2 ± 0.07, P = 0.01, effect size = 0.06) (see Table 1 and Fig. 1; ROC curves are provided in Fig. S1).
TABLE 1.
CSF levels for monoamine metabolites in the PPMI cohort
| HC (n = 56) | PD (n = 95) | Difference PD‐HC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean ± SD | <LOD | ICC | Mean ± SD | <LOD | Estimate | P Value | AUC | Specificity | Sensitivity |
| 3‐Methoxytyrosine | 1.33 ± 0.28 | 0.00 | 0.58 | 2.79 ± 2.11 | 0.00 | −0.02 | 0.64 | 0.54 | 0.00 | 1.00 |
| DOPAC | −1.10 ± 0.41 | 0.00 | 0.74 | −0.99 ± 0.77 | 0.00 | −0.20 | 0.011 | 0.63 | 0.23 | 0.89 |
| DOPA | −0.31 ± 0.25 | 0.00 | 0.63 | 0.59 ± 1.42 | 0.00 | −0.01 | 0.85 | 0.53 | 0.00 | 1.00 |
| DOPEG | 0.49 ± 0.32 | 0.00 | 0.70 | 0.50 ± 0.37 | 0.00 | 0.04 | 0.50 | 0.53 | 0.00 | 1.00 |
| HMPG | 2.07 ± 0.23 | 0.00 | 0.47 | 2.00 ± 0.25 | 0.00 | −0.04 | 0.51 | 0.57 | 0.00 | 1.00 |
| 4‐Hydroxy‐3‐methoxymandelic acid | −0.14 ± 0.34 | 0.00 | 0.69 | −0.13 ± 0.33 | 1.31 | −0.02 | 0.84 | 0.54 | 0.00 | 1.00 |
| 5‐HIAA | 3.71 ± 0.45 | 0.00 | 0.74 | 3.53 ± 0.45 | 0.00 | −0.12 | 0.076 | 0.59 | 0.07 | 0.99 |
| Dopamine | −4.58 ± 0.41 | 2.38 | 0.61 | −4.19 ± 0.61 | 2.62 | 0.15 | 0.072 | 0.61 | 0.13 | 0.96 |
| Histamine | −2.56 ± 0.44 | 18.45 | 0.19 | −2.32 ± 0.95 | 24.02 | 0.25 | 0.077 | 0.58 | 0.02 | 0.93 |
| HVA | 3.85 ± 0.42 | 0.00 | 0.71 | 3.74 ± 0.60 | 0.00 | −0.33 | <0.0001 | 0.71 | 0.43 | 0.87 |
| Noradrenaline (norepinephrine) | −1.52 ± 0.46 | 0.00 | 0.69 | −1.47 ± 0.44 | 0.00 | 0.08 | 0.48 | 0.56 | 0.00 | 1.00 |
| Normetanephrine | −2.28 ± 0.43 | 0.00 | 0.71 | −2.27 ± 0.43 | 0.00 | 0.02 | 0.76 | 0.47 | 1.00 | 0.00 |
| (DOPEG + NM + HMPG + NA)/dopamine | 7.22 ± 0.39 | 0.02 | n/a | 6.78 ± 0.56 | 0.03 | −0.17 | 0.013 | 0.64 | 0.24 | 0.93 |
| DOPAC/dopamine | 3.49 ± 0.43 | 0.02 | n/a | 3.22 ± 0.55 | 0.03 | −0.35 | <0.0001 | 0.71 | 0.38 | 0.84 |
| Dopamine/DOPA | −4.28 ± 0.41 | 0.02 | n/a | −4.82 ± 1.10 | 0.03 | 0.16 | 0.086 | 0.62 | 0.07 | 0.96 |
| DOPEG/noradrenaline | 2.01 ± 0.42 | 0.00 | n/a | 1.97 ± 0.38 | 0.00 | −0.05 | 0.45 | 0.55 | 0.00 | 1.00 |
| HMPG/noradrenaline | 3.59 ± 0.40 | 0.00 | n/a | 3.47 ± 0.38 | 0.00 | −0.13 | 0.082 | 0.61 | 0.07 | 0.95 |
| HVA/dopamine | 8.44 ± 0.45 | 0.02 | n/a | 7.95 ± 0.50 | 0.03 | −0.48 | <0.0001 | 0.77 | 0.47 | 0.85 |
| Noradrenaline/DOPA | −1.21 ± 0.43 | 0.00 | n/a | −2.06 ± 1.46 | 0.00 | 0.09 | 0.17 | 0.58 | 0.02 | 0.99 |
| Normetanephrine/noradrenaline | −0.76 ± 0.23 | 0.00 | n/a | −0.79 ± 0.28 | 0.00 | −0.07 | 0.31 | 0.55 | 0.00 | 1.00 |
Monoamine metabolite levels in PPMI HC and PD CSF samples: levels (log2 transformed), percentages <LOD, ICCs to assess stability between baseline, 1‐year follow‐up, and 2‐year follow‐up in the PPMI HC group, HC‐PD group differences with corresponding AUC and receiver operating characteristics curves, and specificity and sensitivity. Boldface reflects significant case–control differences after multiple testing correction.
CSF, cerebrospinal fluid; PPMI, Parkinson's Progressive Markers Initiative; HC, healthy control; PD, Parkinson's disease; SD, standard deviation; LOD, below the limit of detection; ICC, intraclass correlation coefficient; AUC, area under the curve; DOPAC, 3,4‐dihydroxyphenylacetic acid; DOPA, 3,4‐dihydroxyphenylalanine; DOPEG; 3,4‐dihydroxyphenylglycol; HMPG, 4‐hydroxy‐3‐methoxyphenylglycol; 5‐HIAA, 5‐hydroxy‐3‐indoleacetic acid; HVA, homovanillic acid; n/a, not applicable.
FIG. 1.
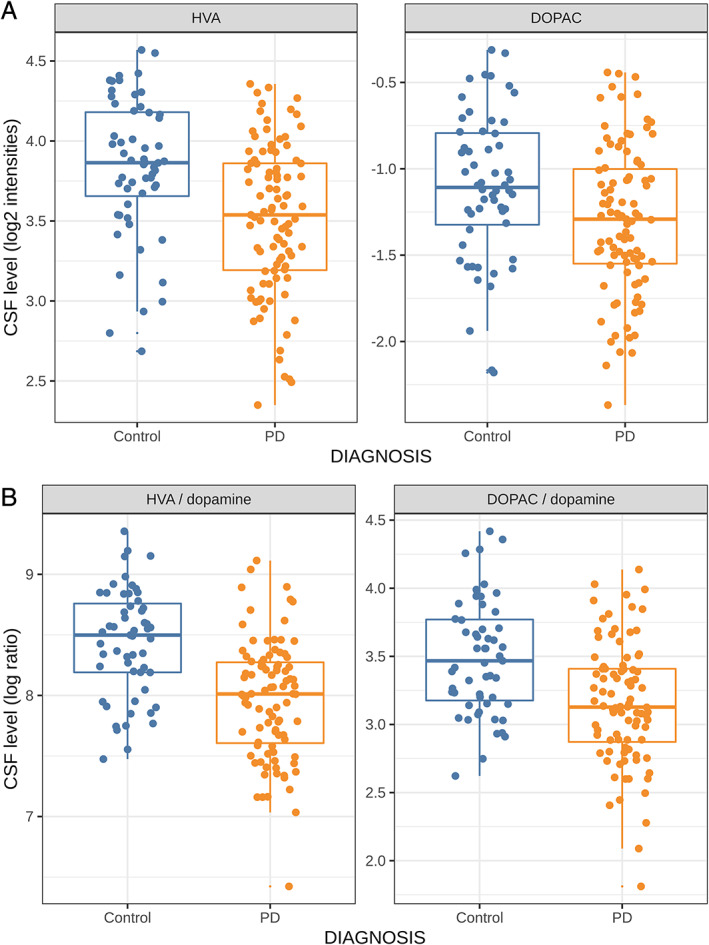
Baseline levels of (A) homovanillic acid (HVA) and 3,4‐dihydroxyphenylacetic acid (DOPAC) and (B) HVA/dopamine and DOPAC/dopamine in healthy control (HC; blue) and Parkinson's disease (PD; orange) Parkinson's Progressive Markers Initiative participants. CSF, cerebrospinal fluid.
In the PPMI cohort, dopamine could be reliably quantified in >97% of the samples, which was supported by test–retest analysis for a subset of samples (see Table S3) and allowed analyses of metabolite ratios. PD CSF levels of HVA correlated with MDS UPDRS total scores (r = −0.26, P < 0.01). Both HVA/dopamine and DOPAC/dopamine correlated with DaT‐SPECT uptake ratios of the mean caudate (both ratios: r = 0.28; P < 0.01) and ipsilateral caudate nucleus (both ratios: r = 0.29, P < 0.01), while raw DOPAC levels correlated with ipsilateral and mean putamen DaT‐SPECT uptake ratios (r = 0.27 and r = 0.28, respectively, both P < 0.01; see Fig. S2).
Long‐Term Within‐Subject Stability
Within‐subject signal stability in longitudinal analyses was assessed by calculating the intraclass correlation coefficient (ICC) for the PPMI HCs at baseline, year 1, and year 2 test values. ICCs ranged from 0.19 (for histamine) to 0.74 (for HIAA), with a median of 0.69 (see Table 1).
Change of Catecholamine Metabolite Levels over Time
No raw or normalized metabolite level changed significantly over 1 year in unmedicated PPMI PD patients (all P > 0.05). Dopaminergic medication affected the levels of DOPA, methoxytyrosine, dopamine, and their respective metabolite ratios (see Fig. S3).
Discussion
This study measured absolute quantities of multiple monoamine metabolites in longitudinal CSF samples from individuals with early PD in the presence and absence of dopaminergic medication.
Various cross‐sectional studies on CSF monoamine metabolites in individuals with PD have been performed.6, 7, 8, 9, 10, 28, 29, 30 However, longitudinal analyses were lacking because the large multicenter DATATOP trial reported no difference in CSF HVA and DOPAC in early PD and during disease progression.7, 11 Longitudinal analyses suffered from high intrapatient variability.11 We hypothesized that multiple factors, such as preanalytical sample processing, site‐to‐site variability,31 and misdiagnoses in PD,32 may have affected the results. Also, the complex analytical method applied may add to the observed variability.12 This study aimed to address these factors with a robust single‐center recruitment (DeNoPa cohort), DaT‐SPECT confirmation of diagnoses in most DeNoPa and all PPMI subjects, and clinical follow‐up and LC–MS/MS method for absolute quantification of metabolites.17, 19
CSF levels of DOPAC and HVA, the end product of dopamine metabolism, were reduced in early PD, confirming previous cross‐sectional studies.8, 9, 10, 28, 29 Correlations observed for dopaminergic metabolites with MDS UPDRS total scores and DaT‐SPECT uptake values support that nigrostriatal neurodegeneration is relevant to early PD and that deficiencies are reflected in CSF.
CSF procedures applied in both studies relied on consensus guidelines and are not necessarily optimized for a given metabolite.33 Thus, absolute values obtained in this study may be affected by ex vivo changes and should be interpreted accordingly. Despite this limitation, the comparably low intrapatient signal variability for a subset of metabolites in longitudinal HC samples is encouraging and supports the future use of this assay in longitudinal studies.
The utility of CSF neurotransmitter metabolite levels to identify prodromal PD or differentiate PD from atypical parkinsonian syndromes remain open questions. Encouraging results from a small prospective cohort study support analysis of CSF monoamine metabolites in prodromal cohorts to identify people who will develop clinical PD.34 Given the proximity of this biomarker panel to the underlying disease pathology, as supported by the present DaT‐SPECT results, it may also identify PD subtypes with diverging neurotransmitter systems deficiencies. Although the present longitudinal data span a relatively short time frame, clinical follow‐up of the PPMI continued since our analysis was performed, and additional information on clinical scales and for various biomarker modalities is available, including their progression with time. We encourage researchers to use our data, which are accessible for downloading, to further deepen our understanding of PD pathophysiology and its progression.
Author Roles
Thomas Kremer: conception, organization, execution, statistical review & critique, writing of the manuscript, and manuscript review & critique.
Kirsten I. Taylor: organization, statistical review & critique, writing of the manuscript, and manuscript review & critique.
Juliane Siebourg‐Polster: statistical analyses, statistical review & critique, writing of the manuscript, and manuscript review & critique.
Thomas Gerken: execution and statistical analyses.
Andreas Staempfli: execution and statistical analyses.
Christian Czech: conception, organization, statistical review & critique, and manuscript review & critique.
Juergen Dukart: statistical analyses, statistical review & critique, and manuscript review & critique.
Sebastian Dziadek: organization, execution, statistical review & critique, and manuscript review & critique.
Gennaro Pagano: statistical review & critique, writing of the manuscript, and manuscript review & critique.
Douglas Galasko: organization and manuscript review & critique.
Tatiana Foroud: organization and manuscript review & critique.
Mark Frasier: conception, organization, and manuscript review & critique.
Lana M. Chahine: execution, writing of the manuscript, and manuscript review & critique.
Christopher S. Coffey: statistical analyses and manuscript review & critique.
Tanya Simuni: manuscript review & critique.
Daniel Weintraub: manuscript review & critique.
John Seibyl: manuscript review & critique.
Kathleen L. Poston: manuscript review & critique.
Arthur W. Toga: manuscript review & critique.
Caroline M. Tanner: manuscript review & critique.
Kenneth Marek: conception, organization, and manuscript review & critique.
Samantha J. Hutten: conception, organization, and manuscript review & critique.
Sebastian Dziadek: manuscript review & critique.
Claudia Trenkwalder: manuscript review & critique.
Brit Mollenhauer: conception, organization, statistical review & critique, writing of the manuscript, and manuscript review & critique.
Financial Disclosures
T.K., K.I.T., J.S.‐P., S.D., and G.P. are full‐time employees of F. Hoffmann–La Roche Ltd. T.K., K.I.T., J.S.‐P., S.D., C.C., and G.P. are stockowners in F. Hoffmann–La Roche Ltd. T.G. is a former employee of Metanomics Health GmbH. C.C. is currently a full‐time employee of Pfizer. S.J.H. is an employee of The Michael J. Fox Foundation for Parkinson's Research (MJFF). D.G. is supported by National Institutes of Health (NIH) grant AGO5131 and by MJFF and has provided consultation for vTv Pharmaceuticals, Eli Lilly, Inc., and Proclara, Inc. T.F. is supported by the NIH and MJFF. L.M.C. receives research support from MJFF, receives research support from the UPMC Competitive Medical Research Fund, is study site investigator for a study sponsored by Biogen, receives research support from the NIH, receives royalties from Elsevier (for authorship), and receives royalties from Wolters Kluwer (for authorship). C.S.C. served as a consultant receiving fees from MJFF; received research funding from the NINDS, NHLBI, and MJFF. D.W. has received research funding or support from MJFF, Alzheimer's Therapeutic Research Initiative (ATRI), Alzheimer's Disease Cooperative Study (ADCS), the International Parkinson and Movement Disorder Society (IPMDS); honoraria for consultancy from Acadia, Aptinyx, Biogen, CHDI Foundation, Clintrex LLC, Enterin, F. Hoffmann–La Roche Ltd., Ferring, Promentis, Signant Health, Sunovion, and Takeda; and license fee payments from the University of Pennsylvania for the QUIP and QUIP‐RS. C.M.T. is an employee of the University of California, San Francisco and the San Francisco Veterans Affairs Health Care System; receives grants from MJFF, the Parkinson's Foundation, the Department of Defense, BioElectron, Roche/Genentech, Biogen Idec, and the NIH; receives compensation for serving on Data Monitoring Committees from Biotie Therapeutics, Voyager Therapeutics, and Intec Pharma; and receives personal fees for consulting from Neurocrine Biosciences, Adamas Therapeutics, Biogen Idec, 23andMe, Alexza, Grey Matter, Acorda, Acadia, and CNS Ratings. J.S. is a consultant to Roche, Invicro, Life Molecular Imaging, Biogen, and LikeMinds and owns equity in Invicro. K.M. has served as a consultant for Pfizer, GE Healthcare, Merck, Lilly, BMS, Piramal, Prothena, Neurophage, nLife, and Roche and receives funding for the following grants: W81XWH‐06‐1‐0678 Establishing an “at risk” cohort for Parkinson Disease Neuroprevention using olfactory testing and DAT imaging, DOD, Investigator 10/1/06–09/30/15; Parkinson Progression Marker Initiative (PPMI), MJFF, Principal Investigator 6/15/09–6/14/18; DAT imaging in LRRK2 family members, MJFF, Principal Investigator 1/15/10–1/14/15; and has ownership in Molecular NeuroImaging, LLC. T.S. has served as a consultant for Acadia, AbbVie, Accorda, Adamas, Allergan, Amneal, Aptinyx, Denali, General Electric (GE), Kyowa, Neuroderm, Neurocrine, Sanofi, Sinopia, Sunovion, Roche, Takeda, Voyager, US World Meds, Parkinson's Foundation, and MJFF for Parkinson's Research; has served as a speaker and received an honorarium from Acadia and Adamas; is on the scientific advisory board for Neuroderm and Sanofi; has received research funding from the NINDS, Parkinson's Foundation, MJFF, Biogen, Roche, Neuroderm, Sanofi, Sun Pharma, AbbVie, IMPAX, and Prevail. B.M. has in the past 12 months received independent research grants from GE Healthcare and honoraria for consultancy from Roche, AbbVie, Biogen, and Sun Pharma Advanced Research Company; is a member of the executive scientific advisory board of MJFF and of the steering committee of the Parkinson Progression Marker Initiative and PI of the Systemic Synuclein Sampling Study of MJFF; and has received grants from the Deutsche Forschungsgemeinschaft (DFG), BMBF, EU (Horizon 2020), Parkinson Fonds Deutschland, Deutsche Parkinson Vereinigung, and MJFF. K.L.P. reports honoraria from invited scientific presentations to universities and professional societies not exceeding $5000/year; is reimbursed by Sanofi, AstraZeneca, and Sangamo BioSciences for the conduct of clinical trials; has received consulting fees from Allergan and Curasen; and is funded by grants from MJFF and the NIH. A.W.T. has no disclosures to report.
Supporting information
Figure S1 Receiver Operating Characteristics (ROC) curves distinguishing HC and PD in DeNoPa (HC: n = 49, PD: n = 62) (upper lane) and PPMI (HC: n = 56, PD: n = 95) (lower lane) participants with respect to baseline levels of log2 transformed metabolites (left panel) and metabolite ratios (right panel).
Figure S2 Pearson's correlations between MDS UPDRS total and Part III scores and DaT‐SPECT uptake ratios of the ipsilateral and contralateral (to clinically most affected side) and mean putamen, caudate nucleus and whole striatum with metabolite and metabolite ratio levels in the baseline PPMI PD cohort. Correlations surviving an uncorrected p < 0.01 are specified with corresponding Pearson's r's.
Figure S3 Metabolite and metabolite ratios over 2 years for HC, PD not taking L‐Dopa (“PD”) and HC and PD taking L‐Dopa (“PD_on_medication”) at baseline and year 1 and 2 PPMI visits.
Table S1 Demographical and clinical characteristics of the DeNoPa (baseline) and PPMI cohort (baseline, year 1, year 2). The number of subjects, age, sex and the MDS UPDRS Part III and total score are included. All are tested for significant differences between the HC and PD treated and untreated groups.
Table S2 Lower and Upper Limits of Detection (LLOD and ULOD, respectively) of LC–MS/MS absolute quantification of metabolites based on DeNoPa and PPMI samples.
Table S3 Test–retest data for the 7 randomly selected blinded CSF samples from the DeNoPa cohort. Each of the samples was measured twice (Test and Retest).
Table S4 Comparison of CSF collection and storage procedures for the DeNoPa and PPMI study.
Table S5 Monoamine metabolite levels in DeNoPa HC and PD CSF samples: levels (log2 transformed), percentages below the limit of detection (<LOD), HC‐PD group differences with corresponding areas and receiver operating characteristics (ROC) curve (AUC), and specificity & sensitivity. Bolding reflects significant case–control differences after multiple testing correction.
Supplementary Statistical Methods
Acknowledgments
This work was supported by The Michael J. Fox Foundation for Parkinson's Research, Abbott, Avid Radiopharmaceuticals, Biogen Idec, Covance, Elan, Eli Lilly and Co, F. Hoffmann–LaRoche Ltd., GE Healthcare, Genentech, Glaxo Smith Kline, Merck and Co., Pfizer Inc., and UCB Pharma SA. The DeNoPa study was supported by unrestricted research grants from the Paracelsus‐Elena‐Klinik, Kassel, Germany, and unrestricted research grants from TEVA Pharma/Lundbeck, Parkinson Fonds Deutschland, The Michael J. Fox Foundation for Parkinson's research, and Deutsche Parkinson Vereinigung. The monoamine metabolite analysis was entirely funded by F. Hoffmann–La Roche. This funder provided support in the form of salaries for authors (T.K., K.I.T., J.S.‐P., A.S., C.C., J.D., S.D., G.P.) but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. PPMI is sponsored by The Michael J. Fox Foundation for Parkinson's Research (MJFF) and is cofunded by MJFF, AbbVie, Avid Radiopharmaceuticals, Biogen Idec, Bristol‐Myers Squibb, Covance, Eli Lilly & Co., F. Hoffman‐La Roche, Ltd., GE Healthcare, Genentech, GlaxoSmithKline, Lundbeck, Merck, MesoScale, Piramal, Pfizer, and UCB. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors thank The Michael J. Fox Foundation, their PPMI colleagues, and the individuals who participated in this study.
Relevant conflicts of interest/Financial disclosures: T.K., J.S.‐P., A.S., S.D., K.I.T., and G.P. are employees of F. Hoffmann–La Roche Ltd. and respective affiliates. T.G. is a former employee of Metanomics Health GmbH. J.D. is a former employee and received consultancy fees from F. Hoffmann–La Roche Ltd. This does not alter our adherence to policies on sharing data and materials.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain 2013;136(8):2419–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3‐hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr 1960;38:1236–1239. [DOI] [PubMed] [Google Scholar]
- 3.Buddhala C, Loftin SK, Kuley BM, Cairns NJ, Campbell MC, Perlmutter JS, et al. Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann Clin Transl Neurol 2015;2(10):949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parnetti L, Castrioto A, Chiasserini D, Persichetti E, Tambasco N, El‐Agnaf O, et al. Cerebrospinal fluid biomarkers in Parkinson disease. Nat Rev Neurol 2013;9(3):131–140. [DOI] [PubMed] [Google Scholar]
- 5.Zetterberg H, Lautner R, Skillbäck T, Rosén C, Shahim P, Mattsson N, et al. CSF in Alzheimer's disease. Adv Clin Chem 2014;65:143–172. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DS, Holmes C, Bentho O, Sato T, Moak J, Sharabi Y, et al. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord 2008;14(8):600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeWitt PA, Galloway MP, Matson W, Milbury P, McDermott M, Srivastava DK, et al. Markers of dopamine metabolism in Parkinson's disease. The Parkinson Study Group. Neurology 1992;42(11):2111–2117. [DOI] [PubMed] [Google Scholar]
- 8.LeWitt P, Schultz L, Auinger P, Lu M. Parkinson study group DATATOP investigators. CSF xanthine, homovanillic acid, and their ratio as biomarkers of Parkinson's disease. Brain Res 2011;1408:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chia L‐G, Cheng F‐C, Kuo J‐S. Monoamines and their metabolites in plasma and lumbar cerebrospinal fluid of Chinese patients with Parkinson's disease. J Neurol Sci 1993;116(2):125–134. [DOI] [PubMed] [Google Scholar]
- 10.Herbert MK, Kuiperij HB, Bloem BR, Verbeek MM. Levels of HVA, 5‐HIAA, and MHPG in the CSF of vascular parkinsonism compared to Parkinson's disease and controls. J Neurol 2013;260(12):3129–3133. [DOI] [PubMed] [Google Scholar]
- 11.Cerebrospinal fluid homovanillic acid in the DATATOP study on Parkinson's disease . Parkinson Study Group. Arch Neurol 1995;52(3):237–245. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrand J, Bourgeois F, Buyse M, Przedborski S, Goldman S. Reproducibility of monoamine metabolite measurements in human cerebrospinal fluid. Acta Neurol Scand 1990;81(5):427–430. [DOI] [PubMed] [Google Scholar]
- 13.Degrell I, Nagy E. Concentration gradients for HVA, 5‐HIAA, ascorbic acid, and uric acid in cerebrospinal fluid. Biol Psychiatry 1990;27(8):891–896. [DOI] [PubMed] [Google Scholar]
- 14.Hartikainen P, Soininen H, Reinikainen KJ, Sirviö J, Soikkeli R, Riekkinen PJ. Neurotransmitter markers in the cerebrospinal fluid of normal subjects. Effects of aging and other confounding factors. J Neural Transm Gen Sect 1991;84(1–2):103–117. [DOI] [PubMed] [Google Scholar]
- 15.Haijes HA, Willemse EAJ, Gerrits J, van der Flier WM, Teunissen CE, Verhoeven‐Duif NM, et al. Assessing the pre‐analytical stability of small‐molecule metabolites in cerebrospinal fluid using direct‐infusion metabolomics. Metabolites 2019;9(10):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noga MJ, Zielman R, van Dongen RM, Bos S, Harms A, Terwindt GM, et al. Strategies to assess and optimize stability of endogenous amines during cerebrospinal fluid sampling. Metabolomics 2018;14(4):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada H, Yamahara A, Yasuda S, Abe M, Oguri K, Fukushima S, et al. Dansyl chloride derivatization of methamphetamine: a method with advantages for screening and analysis of methamphetamine in urine. J Anal Toxicol 2002;26(1):17–22. [DOI] [PubMed] [Google Scholar]
- 18.Kamlage B, Maldonado SG, Bethan B, Peter E, Schmitz O, Liebenberg V, et al. Quality markers addressing preanalytical variations of blood and plasma processing identified by broad and targeted metabolite profiling. Clin Chem 2014;60(2):399–412. [DOI] [PubMed] [Google Scholar]
- 19.Czech C, Berndt P, Busch K, Schmitz O, Wiemer J, Most V, et al. Metabolite profiling of Alzheimer's disease cerebrospinal fluid. PloS One 2012;7(2):e31501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollenhauer B, Trautmann E, Sixel‐Döring F, Wicke T, Ebentheuer J, Schaumburg M, et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013;81(14):1226–1234. [DOI] [PubMed] [Google Scholar]
- 21.Mollenhauer B, Zimmermann J, Sixel‐Döring F, Focke NK, Wicke T, Ebentheuer J, et al. Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology 2016;87(2):168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang J‐H, Irwin DJ, Chen‐Plotkin AS, Siderowf A, Caspell C, Coffey CS, et al. Association of cerebrospinal fluid β‐amyloid 1‐42, T‐tau, P‐tau181, and α‐synuclein levels with clinical features of drug‐naive patients with early Parkinson disease. JAMA Neurol 2013;70(10):1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, et al. The Parkinson progression marker initiative (PPMI). Prog Neurobiol 2011;95(4):629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Parkinson's Progression Marker Initiative , Kang J‐H, Mollenhauer B, Coffey CS, Toledo JB, Weintraub D, et al. CSF biomarkers associated with disease heterogeneity in early Parkinson's disease: the Parkinson's progression markers initiative study. Acta Neuropathol (Berl) 2016;131(6):935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollenhauer B, El‐Agnaf OMA, Marcus K, Trenkwalder C, Schlossmacher MG. Quantification of α‐synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomark Med 2010;4(5):683–699. [DOI] [PubMed] [Google Scholar]
- 26.Kang J‐H. Cerebrospinal fluid amyloid β1‐42, tau, and alpha‐Synuclein predict the heterogeneous progression of cognitive dysfunction in Parkinson's disease. J Mov Disord 2016;9(2):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walk TB, Dostler M. Massenspektrometrisches verfahren zur analyse von substanzgemischen [Internet]. WO2003073464A1, 2003 [Cited 2021 Feb 28]. https://patents.google.com/patent/WO2003073464A1/en/und
- 28.Eldrup E, Mogensen P, Jacobsen J, Pakkenberg H, Christensen NJ. CSF and plasma concentrations of free norepinephrine, dopamine, 3,4‐dihydroxyphenylacetic acid (DOPAC), 3,4‐dihydroxyphenylalanine (DOPA), and epinephrine in Parkinson's disease. Acta Neurol Scand 1995;92(2):116–121. [DOI] [PubMed] [Google Scholar]
- 29.Stefani A, Pierantozzi M, Olivola E, Galati S, Cerroni R, D'Angelo V, et al. Homovanillic acid in CSF of mild stage Parkinson's disease patients correlates with motor impairment. Neurochem Int 2017;105:58–63. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DS, Holmes C, Sharabi Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson's disease and other synucleinopathies. Brain J Neurol 2012;135(Pt 6):1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewczuk P, Beck G, Esselmann H, Bruckmoser R, Zimmermann R, Fiszer M, et al. Effect of sample collection tubes on cerebrospinal fluid concentrations of tau proteins and amyloid beta peptides. Clin Chem 2006;52(2):332–334. [DOI] [PubMed] [Google Scholar]
- 32.Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver‐Dunckley E, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology 2014;83(5):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.del Campo M, Mollenhauer B, Bertolotto A, Engelborghs S, Hampel H, Simonsen AH, et al. Recommendations to standardize preanalytical confounding factors in Alzheimer's and Parkinson's disease cerebrospinal fluid biomarkers: an update. Biomark Med 2012;6(4):419–430. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein DS, Holmes C, Lopez GJ, Wu T, Sharabi Y. Cerebrospinal fluid biomarkers of central dopamine deficiency predict Parkinson's disease. Parkinsonism Relat Disord 2018;50:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Receiver Operating Characteristics (ROC) curves distinguishing HC and PD in DeNoPa (HC: n = 49, PD: n = 62) (upper lane) and PPMI (HC: n = 56, PD: n = 95) (lower lane) participants with respect to baseline levels of log2 transformed metabolites (left panel) and metabolite ratios (right panel).
Figure S2 Pearson's correlations between MDS UPDRS total and Part III scores and DaT‐SPECT uptake ratios of the ipsilateral and contralateral (to clinically most affected side) and mean putamen, caudate nucleus and whole striatum with metabolite and metabolite ratio levels in the baseline PPMI PD cohort. Correlations surviving an uncorrected p < 0.01 are specified with corresponding Pearson's r's.
Figure S3 Metabolite and metabolite ratios over 2 years for HC, PD not taking L‐Dopa (“PD”) and HC and PD taking L‐Dopa (“PD_on_medication”) at baseline and year 1 and 2 PPMI visits.
Table S1 Demographical and clinical characteristics of the DeNoPa (baseline) and PPMI cohort (baseline, year 1, year 2). The number of subjects, age, sex and the MDS UPDRS Part III and total score are included. All are tested for significant differences between the HC and PD treated and untreated groups.
Table S2 Lower and Upper Limits of Detection (LLOD and ULOD, respectively) of LC–MS/MS absolute quantification of metabolites based on DeNoPa and PPMI samples.
Table S3 Test–retest data for the 7 randomly selected blinded CSF samples from the DeNoPa cohort. Each of the samples was measured twice (Test and Retest).
Table S4 Comparison of CSF collection and storage procedures for the DeNoPa and PPMI study.
Table S5 Monoamine metabolite levels in DeNoPa HC and PD CSF samples: levels (log2 transformed), percentages below the limit of detection (<LOD), HC‐PD group differences with corresponding areas and receiver operating characteristics (ROC) curve (AUC), and specificity & sensitivity. Bolding reflects significant case–control differences after multiple testing correction.
Supplementary Statistical Methods


