Summary
Background
Dupilumab was equally effective among all racial subgroups in clinical trials, but a direct comparison in daily practice is lacking.
Objectives
To investigate the effectiveness of dupilumab in patients with atopic dermatitis (AD) in the Netherlands and Japan over 80 weeks of treatment.
Methods
A longitudinal comparative cohort study was conducted in patients with AD who were treated with dupilumab in daily practice. We used linear mixed‐effects models to determine changes over time.
Results
We found statistically significant differences in sex, disease onset, body mass index and therapeutic history between Dutch (n = 208) and Japanese (n = 153) patients. The baseline Eczema Area and Severity Index (EASI) score was higher in Japanese patients (23·8 vs. 14·8), while baseline Patient‐Reported Outcome Measures (PROMs) were higher in Dutch patients. EASI scores decreased quickly to a level indicating ‘mild disease’ (EASI < 7), and remained low in both countries. However, PROMs showed different trajectories with better scores in Japan.
Conclusions
Dupilumab showed significant, comparable and sustained improvement of EASI scores in Japanese and Dutch patients. However, we found striking differences in the effect on PROMs between the countries, with a better outcome in Japanese patients.
Short abstract
What is already known about this topic?
In clinical trials, dupilumab was found to be equally effective among all racial subgroups; however, a direct comparison in daily practice between patients with atopic dermatitis (AD) in Japan and the Netherlands is currently lacking.
Although phenotypical and immunological differences between races are described for AD, literature about the racial differences in coping mechanisms and the perception of disease in AD is lacking.
What does this study add?
This study showed a significant improvement of disease severity in both Japanese and Dutch patients with AD during dupilumab treatment in daily practice.
In addition to racial disease‐specific differences, healthcare system‐ or culture‐related characteristics might contribute to differences in physician‐ and patient‐reported outcome measures (PROMs) between countries.
What are the clinical implications of the work?
Consideration of PROMs and healthcare system‐ or culture‐related characteristics, in addition to disease severity measures including the Eczema Area and Severity Index, is of added value in the evaluation of treatment effectiveness in clinical studies and in daily practice.
Linked Comment: A. Taïeb and N. Katoh. Br J Dermatol 2021; 185:479–480.
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease. The prevalence of AD in diverse ethnic groups shows wide ranges (1–25%), with a higher prevalence reported in Asians compared with Europeans.1, 2 Although individuals with any ethnicity and skin type can be affected, phenotypical and immunological differences due to environmental factors, pigmentation, distinct T‐helper (Th) cell profiles, epidermal structure and presence of FLG mutations have been described.2, 3, 4, 5 Recently, Koga et al.6 found a stronger Th17/Th22 polarization and a more blended phenotype with features of both AD and psoriasis in Asian patients with AD compared with Europeans.4, 6 Until now, literature about the racial differences in coping mechanisms and the perception of disease in AD is lacking.
In most countries, topical corticosteroids (TCS), topical calcineurin inhibitors and emollients represent the first step in the treatment of AD. In Japan (JP), serum thymus and activation‐regulated chemokine (TARC/CCL17) levels have been used widely as a biomarker for disease severity and as a tool for ‘tight (disease) control’ since 2008, especially during topical treatment in daily practice. TARC is a chemokine that plays a role in attracting inflammatory cells to the skin. After expression on the vascular endothelium, TARC is released in blood, resulting in measurable levels reflecting disease severity.7 In about 15% of patients with AD, topical treatment is insufficiently effective and systemic treatments including ciclosporin A (CsA), methotrexate (MTX), azathioprine, mycophenolic acid (MPA), mycophenolate mofetil (MMF) and dupilumab are indicated.8 In the Netherlands (NL), dupilumab treatment is reimbursed (100%) when a patient has been treated with at least one systemic immunosuppressant.9 In JP, dupilumab treatment is indicated (70% reimbursed) for patients with moderate‐to‐severe AD not controlled despite adequate topical treatment for ≥ 6 consecutive months, not necessarily limited by prior systemic therapies. Systemic immunosuppressants are less frequently prescribed in JP. Prescription of MTX, MPA and MMF for AD is not allowed, but short courses of CsA (maximum of 12 weeks) or oral corticosteroids are used.1 In clinical trials, dupilumab was found to be equally effective among all racial subgroups.1, 10 However, a direct comparison of the effectiveness of dupilumab and other systemic therapies in daily practice in patients with AD in JP and NL is currently lacking.3
In both Osaka (JP) and Rotterdam (NL), treatment effect and disease severity in daily practice is assessed using physicians’ and patients’ observations and experiences supported by clinical scores including Eczema Area and Severity Index (EASI), Patient‐Oriented Eczema Measure (POEM), Numeric Rating Scale (NRS)‐pruritus and Dermatology Life Quality Index (DLQI). In addition, serum TARC levels were measured in Japanese patients.
Here, we aim to investigate the effectiveness of dupilumab treatment in patients with AD in the Netherlands compared with Japan.
Patients and methods
We conducted a longitudinal comparative cohort study, using data that was prospectively collected in daily practice at the Erasmus MC University Medical Center (Rotterdam, NL) and the Osaka Habikino Medical Center (Habikino, Osaka, JP) from October 2017 until June 2020.
All patients with AD (aged ≥ 13 years in NL, ≥ 15 years in JP) who started dupilumab treatment were informed about the collection of data and patients who gave consent to publish pseudonymized information relating to them were consecutively included. In the Erasmus MC, data were collected as part of the Immune‐Mediated Inflammatory Disorders (IMID) Registry. Dupilumab was used according to the product label and interval deviations and use of concomitant systemic immunosuppressants were recorded.11 Additionally, patients were encouraged to continue the use of topical therapy.
Data were collected at the start of dupilumab treatment, after 4 weeks (NL), after 3–4 months of treatment and every 3–4 months thereafter. Patient characteristics, therapeutic history and current AD treatment were recorded at baseline. Disease severity, symptoms and the impact on quality of life were assessed at every visit using EASI (0–72), POEM (0–28), DLQI (0–30) and NRS‐pruritus (0–10). The peak pruritus score of the past 7 days was recorded in JP, whereas the peak pruritus score of the past 24 h was recorded in NL. Blood samples were collected from all Japanese patients at all visits in order to measure serum biomarker levels (i.e. TARC). Side‐effects were not recorded in a standardized manner in JP and therefore this is beyond the scope of this study.
Data analysis
Because data were collected in daily practice, follow‐up schemes show a variance in timing of visits. We used linear mixed‐effects (LME) models to evaluate the effectiveness of dupilumab and to describe and present the change of the repeatedly measured, continuous score of interest in time (days since start of treatment). We analysed measurements done at visits from the start up to 80 weeks of treatment. We used natural cubic splines in both the fixed and random‐effects models to capture the nonlinear evolution over time. Sex, age and country were included as covariates in our model, including an interaction of time and country. Please see Methods S1 in the Supporting Information for additional information.
Ethical approval
Our study was exempted from evaluation by the local Medical Research Ethics Committees (NL: MEC‐2017‐1123, W18_097#18·123; JP: OHMC‐MEC‐1067). The study was conducted in accordance with STROBE recommendations.
Results
In total, 361 patients with AD (NL, n = 208; JP, n = 153) were consecutively included. As shown in Table 1, the majority of the patients were male with a statistically significant difference in proportion between NL (53%) and JP (85%) (P < 0·001). The median age at the time of initiation of dupilumab was 33 (IQR 24–51; NL) and 34 (IQR 21–45; JP) years, with a younger onset of AD in NL compared with JP (P < 0·001). Atopic comorbidities were more frequently reported in NL (65–77%) than in JP (41–65%), and a statistically significant lower body mass index (BMI) (24·9 vs. 22·9) was found in JP. About 90% of the Dutch patients had used CsA compared with less than 50% of the Japanese patients. These differences between countries were also found for MTX and MPA/MMF. Additionally, we found a difference in the use of concomitant systemic immunosuppressants in the transition to dupilumab treatment between the countries (NL: 124 of 208; JP: 58 of 153) (Table 1; for further details regarding NL see Table S1; see Supporting Information). Immunosuppressants were tapered and discontinued in 111 of 124 Dutch and 53 of 58 Japanese patients, after a median of 85 [NL, interquartile range (IQR) 42–167] and 56 (JP, IQR 28–133) days of treatment. The remaining patients (NL, n = 13; JP, n = 5) were still using immunosuppressants at the time of analysis. A subset of the Japanese patients (n = 7) was treated intensively with TCS during a clinical admission in the hospital just before starting dupilumab treatment.
Table 1.
Demographics and clinical characteristics at baselinea
| Characteristic | NL (n = 208)b | JP (n = 153)b | P‐value |
|---|---|---|---|
| Male sex | 111 (53) | 130 (85) | < 0·001* |
| Age of onset ADc | < 0·001* | ||
| 0 ≤ 2 years | 116 (56) | 65 (43) | |
| 2 ≤ 6 years | 21 (10) | 29 (19) | |
| 6 ≤ 18 years | 21 (10) | 35 (23) | |
| ≥ 18 years | 34 (16) | 23 (15) | |
| Age at start of dupilumab, median (IQR), years | 33 (24–51) | 34 (21–45) | 0·016* |
| Number of adolescents (< 18 years) | 4 (2) | 24 (16) | < 0·001* |
| Raced | < 0·001*,m | ||
| White | 163 (78) | – | |
| Black | 14 (7) | – | |
| Asian | 11 (5) | 153 (100) | |
| Other | 2 (1)n | – | |
| Fitzpatrick skin type | < 0·001*,o | ||
| I | 4 (2) | – | |
| II | 144 (69) | – | |
| III | 22 (11) | 71 (46) | |
| IV | 16 (8) | 81 (53) | |
| V | 18 (9) | 1 (1) | |
| VI | 4 (2) | – | |
| Atopic/allergic conditionsp | |||
| Asthmae | 135 (65) | 63 (41) | < 0·001* |
| Allergic (rhino)conjunctivitise | 161 (77) | 100 (65) | 0·009* |
| Allergic contact dermatitisf | 98 (47) | 1 (1) | < 0·001* |
| BMI, median (IQR)g | 24·9 (22·2–27·9) | 22·9 (20·3–26·5) | < 0·001* |
| Family history of atopic diseasesh | 150 (72) | 101 (66) | 0·381 |
| Previous use of phototherapyi | 126 (61) | 35 (23) | < 0·001* |
| Number of previous used conventional systemic immunosuppressants | < 0·001*,o | ||
| 0 | 7 (3) | 83 (54) | |
| 1 | 64 (31) | 70 (46) | |
| 2 | 98 (47) | – | |
| ≥ 3 | 39 (19) | – | |
| Previously used conventional systemic immunosuppressants | |||
| Ciclosporin Aj | 187 (90) | 70 (46) | < 0·001* |
| Methotrexatek | 77 (37) | – | < 0·001* |
| Azathioprine | 39 (19) | 3 (2) | < 0·001* |
| MPA/MMF | 78 (38) | – | < 0·001* |
| Therapy until start of dupilumab/continued after start of dupilumabl | |||
| Ciclosporin A | 61 (29)/59 (97) | 58 (37)/58 (100) | < 0·001*/< 0·001* |
| Methotrexate | 23 (11)/11 (48) | – | < 0·001*/NA |
| Azathioprine | 12 (6)/9 (75) | 1 (1)/1 (100) | 0·009*/1·0 |
| MPA/MMF | 18 (9)/17 (94) | – | < 0·001*/NA |
| Otherq | 29 (14)/28 (97) | 1 (1)/– | < 0·001*/< 0·001* |
| None | 65 (31)/NA | 93 (61)/NA |
AD, atopic dermatitis; BMI, body mass index; IQR, interquartile range; MMF, mycophenolate mofetil; MPA, mycophenolic acid; NL, the Netherlands; JP, Japan
Statistical tests used: for proportions: Chi‐squared test [Expected (E) < 5; Fisher’s exact test); for distributions across JP / NL: Mann–Whitney U‐test. *P < 0·05, statistical significance. bAll values n (%), unless otherwise noted. c–lMissing values: c n = 16 (NL), n = 1 (JP); d n = 18 (NL); e n = 1 (NL); f n = 51 (NL); g n = 31 (NL); h n = 15 (NL), n = 13 (JP); i n = 52 (NL); j n = 2 (JP); k n = 1 (JP); l n = 1 (NL). mCategories combined into white and other, in order to be able to conduct statistic tests. nIndian, n = 2. oCategories combined into I + II vs. III–VI (skin type) and 0 vs. ≥ 1 (previous therapies), in order to be able to conduct statistic tests (more than two categories and several cells with < 5 counts, Fisher's exact only possible as 2 × 2). pPatient‐reported or physician‐diagnosed. qOral corticosteroids: NL, n = 27 NL, n = 1 JP; alitretinoin n = 2 NL.
In our cohort, 61% of the patients (NL, n = 122; JP, n = 98) were treated with dupilumab according to the product label [once every 2 weeks (Q2W)] during total follow‐up. Eight per cent of the patients (NL, n = 29; JP, n = 0) were (temporarily) treated in a shortened interval (< 2 weeks) and 25% of the patients was treated in an extended interval (NL, n = 35; JP, n = 55), which was initiated after 1 year of Q2W application in JP. Thirty‐three (NL, n = 20; JP, n = 13) patients discontinued dupilumab treatment due to insufficient effectiveness (NL, n = 10; JP, n = 1), side‐effects (NL, n = 4), a combination of insufficient effectiveness and side‐effects (NL, n = 4), financial considerations (JP, n = 11), changed diagnosis (JP, n = 1) or anticipated pregnancy (NL, n = 2). No evident common phenotypical characteristics, laboratory markers or other predictors of failure could be detected in these patients.
The disease course from baseline until 80 weeks (= 560 days) of treatment is shown in Figure 1a–e. The number of unique patients with recorded EASI scores at specific time points ranged from 194 (NL) and 145 (JP) at the start of dupilumab treatment, to 103 (NL) and 85 (JP) at 1 year, and subsequently 44 (NL) and 38 (JP) at 1·5 years of treatment (Tables S2 and S3; see Supporting Information).
Figure 1.
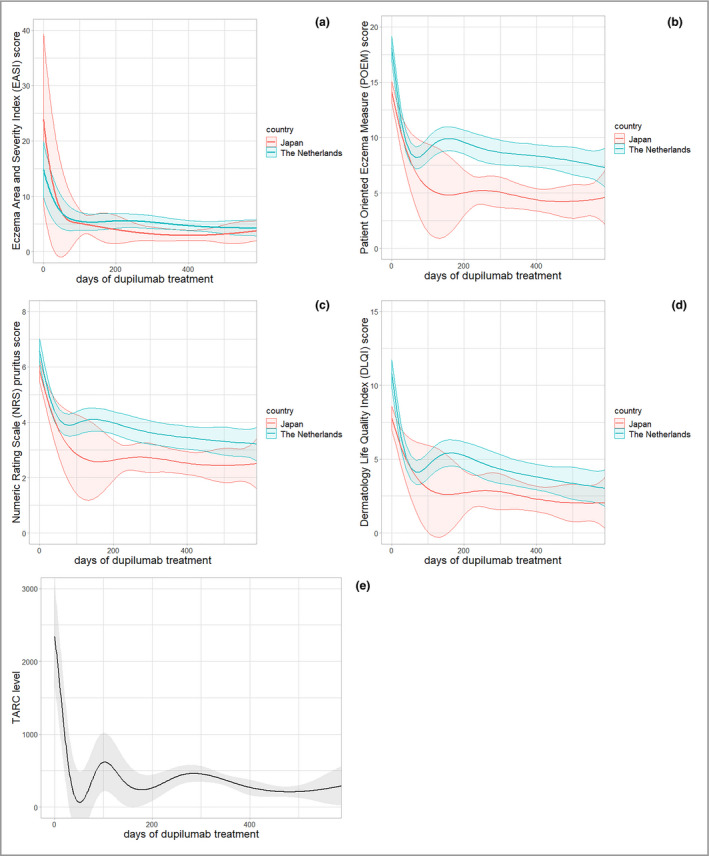
Effectiveness of dupilumab in patients with atopic dermatitis: outcome measures over time during dupilumab treatment. All outcome measures have shown clinically relevant improvement with at least one unit of minimal clinically important difference. (a) The EASI score shows a rapid decrease with comparable trajectories in both countries, while Patient‐Reported Outcome Measures (PROMs) [(b) Patient‐Oriented Eczema Measure and (c) Numeric Rating Scale (NRS)‐Pruritus] showed different trajectories with relatively high scores and a clinically relevant, better outcome for PROMs in JP patients. (c) NRS‐Pruritus shows peak pruritus past 24 h (NL) and mean pruritus past 7 days (JP). (d) DLQI score in time during dupilumab treatment. (e) TARC levels after starting dupilumab treatment (in JP patients). Bands show 95% confidence intervals
The estimated severity scores (Table 2) indicate moderate‐to‐severe AD at baseline. Interestingly, although the physician‐reported severity scores were higher in Japanese patients (i.e. EASI 23·8 vs. 14·8 in NL), all patient‐reported outcome measures (PROMs) indicated worse disease in NL. A statistically significant difference in estimated baseline scores between JP and NL was found for POEM (P = < 0·001), DLQI (P = < 0·001) and NRS‐pruritus (P = 0·022), while a minimal clinically important difference (MCID) between both populations at baseline was observed for EASI (actual difference 9·0; MCID 6·6) and POEM (actual difference 4·0; MCID 3·4). The baseline TARC level, which was measured only in JP, was estimated to be 2239 pg mL–1 (95% CI 1553–2925) (maximum reference value 450 pg mL–1).
Table 2.
Effectiveness of dupilumab: estimated (clinical) severity scores (ESS) over time
| The Netherlands | Japan | |||
|---|---|---|---|---|
| ESS (95% CI) | % change from baseline | ESS (95% CI) | % change from baseline | |
| Eczema Area and Severity Index score | ||||
| Baseline | 14·8 (9·9–19·7) | – | 23·8 (8·6–39·0) | – |
| 4 weeks | 9·4 (5·8–13·0) | –36·5 | 11·6 (0·6–22·6) | –51·3 |
| 12 weeks | 5·7 (3·9–7·5) | –61·5 | 5·3 (1·4–9·2) | –77·7 |
| 52 weeks | 4·9 (4·0–5·8) | –66·9 | 3·0 (2·0–4·0) | –87·4 |
| 80 weeks | 4·3 (2·9–5·7) | –70·9 | 3·6 (1·8–5·4) | –84·9 |
| Patient‐Oriented Eczema Measure score | ||||
| Baseline | 18·1 (17·0–19·2) | – | 14·1 (13·2–15·0) | – |
| 4 weeks | 11·3 (10·3–12·3) | –37·6 | 10·2 (8·7–11·7) | –27·7 |
| 12 weeks | 8·4 (7·4–9·4) | –53·6 | 6·0 (2·6–9·4) | –57·4 |
| 52 weeks | 8·4 (7·4–9·4) | –53·6 | 4·6 (3·4–5·8) | –67·4 |
| 80 weeks | 7·5 (6·3–8·7) | –58·6 | 4·4 (2·9–5·9) | –68·8 |
| Dermatology Life Quality Index score | ||||
| Baseline | 10·8 (9·9–11·7) | – | 7·8 (7·0–8·6) | – |
| 4 weeks | 6·3 (5·5–7·1) | –41·7 | 5·7 (4·5–6·9) | –26·9 |
| 12 weeks | 4·2 (3·4–5·0) | –61·1 | 3·3 (0·8–5·8) | –57·7 |
| 52 weeks | 4·0 (3·1–4·9) | –63·0 | 2·5 (1·5–3·5) | –67·9 |
| 80 weeks | 3·1 (2·0–4·2) | –71·3 | 2·0 (0·8–3·2) | –74·4 |
| Numeric Rating Scale‐pruritus | ||||
| Baseline | 6·6 (6·2–7·0) | – | 5·9 (5·5–6·3) | – |
| 4 weeks | 4·9 (4·5–5·3) | –25·8 | 4·5 (3·9–5·1) | –23·7 |
| 12 weeks | 3·9 (3·5–4·3) | –40·9 | 3·0 (1·8–4·2) | –49·2 |
| 52 weeks | 3·5 (3·1–3·9) | –47·0 | 2·6 (2·1–3·1) | –55·9 |
| 80 weeks | 3·2 (2·7–3·7) | –51·5 | 2·5 (1·9–3·1) | –57·6 |
| Thymus and activation‐regulated chemokine (TARC) levels a | ||||
| Baseline | – | – | 2239 (1553–2925) | – |
| 4 weeks | – | – | 578 (80–1076) | –75·3 |
| 12 weeks | – | – | 483 (87–878) | –79·4 |
| 52 weeks | – | – | 339 (259–419) | –85·5 |
| 80 weeks | – | – | 255 (49–461) | –89·1 |
CI, confidence interval.
Measured in pg mL–1.
After 12 weeks of treatment, we observed an estimated mean EASI of 5·7 and an absolute change from baseline (Δ) of –9·1 (–61·5%) in NL and 5·3 (Δ –18·5; –77·7%) in JP; a mean POEM of 8·4 (Δ –9·7, –53·6%) in NL and 6·0 (Δ –8·1, –57·4%) in JP; a mean DLQI of 4·2 (Δ –6·6, –61·1%) in NL and 3·3 (Δ –4·5, –57·7%) in JP; and a mean NRS‐pruritus of 3·9 (Δ –2·7, –40·9%) in NL and 3·0 (Δ –2·9, –49·2%) in JP (Table 2). All outcome measures have shown improvement with at least one MCID unit. As shown in Figure 1 (b,c), all PROMs in Dutch patients show an increase after 12–16 weeks of treatment, which was not reflected by the EASI score (Figure 1a). A continued improvement of PROMs from about 21 weeks (approx. 150 days) until approximately 70–80 weeks (about 490–560 days) is observed in both countries. We found statistically significant differences in POEM scores from about 145 days of treatment until the end of follow‐up between JP and NL patients; DLQI scores from about 200 to 230 days of treatment; and NRS‐pruritus scores from about 175 to 275 days, and 330 to 465 days of treatment (Figure 1a–d). After 80 weeks (560 days) of treatment, we found a difference between the countries in all outcome measures, with better outcomes in JP compared with NL patients, as shown in Table 2, respectively: EASI 0·7, POEM 3·1, DLQI 1·1, NRS 0·7).
Discussion
In this longitudinal comparative cohort study, we evaluated the effectiveness of dupilumab in Dutch and Japanese patients with AD in daily practice. Dupilumab showed significant improvement on all outcome measures in both Japanese and Dutch patients. Although the mean estimated baseline EASI score was higher in Japanese patients, baseline PROMs were significantly higher in Dutch patients. The mean estimated EASI scores of Dutch and Japanese patients decreased quickly to a score indicating ‘mild disease’, with a comparable trajectory from 12 weeks until the end of follow‐up. Strikingly, PROMs showed different trajectories with relatively high levels and clinically relevant, better outcomes for PROMs in Japanese patients.
Interestingly, we found large differences in demographics and clinical characteristics of Japanese compared with Dutch patients at baseline. We found a higher number of male patients in JP (85%, < 0·001), a later onset of disease in Japanese patients (< 0·001) and lower BMI (< 0·001) compared with Dutch patients. This is partly in line with data from an observational registry of adult Japanese patients with AD and phase III trial data, reporting 56–80% male patients with moderate‐to‐severe AD, and a median onset of AD of 3–4 years of age.1, 12 However, a study conducted by the Japanese Dermatological Association including all disease severity categories concluded that there is no sex difference in the incidence of AD.13 This might suggest a higher risk for developing severe AD in male patients; however, literature on this topic is lacking. Kato et al. found significant negative correlations between BMI and EASI reduction in the long term, suggesting that dupilumab might be less effective in obese patients.14 However, the addition of BMI as a covariate in our analyses did not result in clinically relevant changes of the outcome. Additionally, we found higher incidences of atopic comorbidities in Dutch patients, which might be explained by the fact that in the Japanese cohort only physician‐diagnosed atopic comorbidities were registered, while in the Dutch cohort patient‐reported conditions were also included. Differences in previous and concomitant use of systemic immunosuppressants can be explained by differences in prescription behaviour and regulations between countries, as discussed earlier. However, we have recently shown that the use of concomitant systemic immunosuppressants does not affect long‐term treatment effectiveness.15
Analysis of our outcome measures showed large confidence intervals for the Japanese patients, as shown in Figure 1a–d. This can be explained by the overrepresentation of males in the Japanese cohort. Additional sensitivity analyses ruled out the influence of this overrepresentation on the differences found between the countries. Factors that may explain these differences include racial disease‐specific differences, differences in healthcare organization and cultural practices, and racial differences in coping and disease experiences. In our study, we found differences in POEM and NRS between the countries. However, differences in DLQI were less evident. This may be because the DLQI is not disease‐specific and includes only questions regarding general physical disabilities. The minor differences in EASI and DLQI scores between countries suggest that cultural differences, e.g. differences in coping, may explain this discrepancy. It is known that cultural characteristics determine the perception of health and illness. Japan is known to have a relatively low absenteeism (i.e. sickness absence) and high presenteeism (i.e. sickness presence) rate compared with other countries, which might reflect a different culture‐related coping strategy as well.16 However, there have been no publications on this topic for AD in particular.17 Additionally, various dimensions of disease or illness perception have been associated with different aspects of the outcome.18 Secondly, the discrepancy might also be the result of the difference in AD healthcare organization. In Japan, focus on concomitant TCS use is expected to be higher compared with NL, possibly resulting in improvement of their disease. This is mainly due to the experience‐based knowledge of accurate remission levels with concomitant TCS based on serum TARC levels.
The rebound effect that was found in the Dutch cohort from approximately 12 weeks of treatment might be the result of a response shift due to (temporal) recalibration.19 This shift might be the result of changing expectations of the patients during (effective) treatment (i.e. patients become more critical during effective therapy).19 It could be speculated that Japanese patients are less susceptible to this response shift, partly because of the gratitude that could possibly be higher due to the personal financial investment that is required during dupilumab treatment in Japan (approximately ¥40000/€317 per month).20 Notably, this rebound phenomenon was reflected by increasing TARC levels in Japanese patients, although levels remained within the reference value that is used in daily practice (< 600 pg mL–1) (Figure 1e). Overall, PROMs showed relatively high levels during follow‐up with a POEM up to 10·0 in NL and 6·0 in JP, which is in line with the available literature.21, 22 This might suggest that consideration of PROMs in addition to disease severity measures including EASI is of added value in the evaluation of treatment effectiveness in studies and daily practice.
The declining ratio between actual and expected measurements, possibly considered a limitation, could be explained by the extended intervals between follow‐up visits in patients with effective treatment (Table S3). Interestingly, the persistence with dupilumab was comparable in JP and NL, despite differences in reimbursement (Table S2). Furthermore, there were minor differences in outcome measures and visit schedules between the countries, but these did not complicate our analyses using the LME models. In addition, side‐effects were not analysed in this study due to the absence of standardized records of side‐effects in daily practice.
This is the first study that directly compares dupilumab treatment in Dutch and Japanese adult patients with AD. Dupilumab showed significant improvement of AD in Japanese and Dutch patients. Although the effect on disease severity (EASI) was similar, we found differences in the effect on PROMs. These differences might be the result of cultural‐ or healthcare system‐related differences.
Author Contribution
Linde Elisabeth Maria de Wijs: Conceptualization (lead); Data curation (lead); Formal analysis (equal); Investigation (lead); Methodology (lead); Project administration (lead); Resources (equal); Software (lead); Validation (lead); Visualization (lead); Writing‐original draft (lead); Writing‐review & editing (lead). R.F.T. Fujimoto: Conceptualization (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Visualization (supporting); Writing‐review & editing (supporting). E.R. Andrinopoulou: Formal analysis (supporting); Methodology (supporting); Writing‐review & editing (supporting). Tamar Nijsten: Conceptualization (supporting); Investigation (supporting); Methodology (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing‐review & editing (supporting). Yoko Kataoka : Conceptualization (supporting); Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (equal); Supervision (equal); Validation (supporting); Visualization (supporting); Writing‐review & editing (equal). DirkJan Hijnen: Conceptualization (supporting); Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (equal); Supervision (equal); Validation (supporting); Visualization (equal); Writing‐review & editing (equal).
Supporting information
Methods S1 Extensive information on data analysis.
Table S1 Use of concomitant systemic immunosuppressants in the Netherlands (n = 124).
Table S2 Number of individual patients with collected data at specific time points.
Table S3 Expected and available number of individual patients with collected data at specific time points.
Acknowledgments
The authors, L.E.M. de Wijs and Y. Kataoka would like to thank the International Society of Atopic Dermatitis (ISAD) for the Research Fellowship Grant that was awarded to L.E.M. de Wijs, and made this collaboration possible.
Funding sources ISAD Research Fellowship grant awarded to L.E.M. de Wijs.
Conflicts of interest L.E.M.d.W.: ISAD Research Fellowship grant. R.F.T.F.: research grants from AbbVie, Eli Lilly, LEO Pharma, Maruho, Otsuka and Pfizer. E.R.A. and T.E.C.N.: none. D.H.: investigator for AbbVie, Galderma, LEO Pharma, MedImmune/AstraZeneca, Novartis and Sanofi/Regeneron; consultancies for Incyte, Janssen, LEO Pharma, Lilly, MedImmune/AstraZeneca, Novartis, Pfizer and Regeneron/Sanofi. Y.K.: lecture honoraria from Sanofi and Sysmex; research grants from AbbVie, Eli Lilly, LEO Pharma, Maruho, Otsuka, Pfizer and Sanofi.
D.H. and Y.K. contributed equally.
References
- 1.Katoh N, Kataoka Y, Saeki Het al. Efficacy and safety of dupilumab in Japanese adults with moderate‐to‐severe atopic dermatitis: a subanalysis of three clinical trials. Br J Dermatol 2020; 183:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunner PM, Guttman‐Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol 2019; 122:449–55. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups – variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol 2018; 27:340–57. [DOI] [PubMed] [Google Scholar]
- 4.Noda S, Suárez‐Fariñas M, Ungar Bet al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol 2015; 136:1254–64. [DOI] [PubMed] [Google Scholar]
- 5.Osawa R, Konno S, Akiyama Met al. Japanese‐specific filaggrin gene mutations in Japanese patients suffering from atopic eczema and asthma. J Invest Dermatol 2010; 130:2834–6. [DOI] [PubMed] [Google Scholar]
- 6.Koga C, Kabashima K, Shiraishi Net al. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol 2008; 128:2625–30. [DOI] [PubMed] [Google Scholar]
- 7.Kataoka Y. Thymus and activation‐regulated chemokine as a clinical biomarker in atopic dermatitis. J Dermatol 2014; 41:221–9. [DOI] [PubMed] [Google Scholar]
- 8.Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol 1998; 139:73–6. [DOI] [PubMed] [Google Scholar]
- 9.NVDV . Introductie van dupilumab voor ernstig constitutioneel eczeem (CE). Available at: https://nvdv.nl/storage/app/media/uploaded‐files/introductie‐van‐dupilumab‐voor‐ernstig‐constitutioneel‐eczeem‐ce‐2018‐standpunt.pdf [in Dutch] (last accessed 19 April 2021).
- 10.Alexis AF, Rendon M, Silverberg JIet al. Efficacy of dupilumab in different racial subgroups of adults with moderate‐to‐severe atopic dermatitis in three randomized, placebo‐controlled phase 3 trials. J Drugs Dermatol 2019; 18:804–13. [PubMed] [Google Scholar]
- 11.Sanofi‐Aventis B.V. Dupilumab – Summary of product characteristics (SPC) 2018.
- 12.Katoh N, Saeki H, Kataoka Yet al. Atopic dermatitis disease registry in Japanese adult patients with moderate to severe atopic dermatitis (ADDRESS‐J): baseline characteristics, treatment history and disease burden. J Dermatol 2019; 46:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi S, Esaki H, Furue M. Epidemiology of atopic dermatitis in Japan. J Dermatol 2014; 41:200–4. [DOI] [PubMed] [Google Scholar]
- 14.Kato A, Kamata M, Ito Met al. Higher baseline serum lactate dehydrogenase level is associated with poor effectiveness of dupilumab in the long term in patients with atopic dermatitis. J Dermatol 2020; 47:1013–9. [DOI] [PubMed] [Google Scholar]
- 15.de Wijs LEM, Bosma AL, Erler NSet al. Effectiveness of dupilumab treatment in 95 patients with atopic dermatitis: daily practice data. Br J Dermatol 2020; 182:418–26. 10.1111/bjd.18179 [DOI] [PubMed] [Google Scholar]
- 16.Chimed‐Ochir O, Nagata T, Nagata Met al. Potential work time lost due to sickness absence and presence among Japanese workers. J Occup Environ Med 2019; 61:682–8. [DOI] [PubMed] [Google Scholar]
- 17.Kaptein AA, Yamaoka K, Snoei Let al. Illness perceptions and quality of life in Japanese and Dutch women with breast cancer. J Psychosoc Oncol 2013; 31:83–102. 10.1080/07347332.2012.741092 [DOI] [PubMed] [Google Scholar]
- 18.Kaptein AA, Yamaoka K, Snoei Let al. Illness perceptions and quality of life in Japanese and Dutch patients with non‐small‐cell lung cancer. Lung Cancer 2011; 72:384–90. [DOI] [PubMed] [Google Scholar]
- 19.Carver CS, Scheier MF. Scaling back goals and recalibration of the affect system are processes in normal adaptive self‐regulation: understanding ‘response shift’ phenomena. Soc Sci Med 2000; 50:1715–22. [DOI] [PubMed] [Google Scholar]
- 20.Tikkanen R, Osborn R, Mossialos Eet al. International Health Care System Profiles. The Commonwealth Fund, 2020. Available from: https://www.commonwealthfund.org/international‐health‐policy‐center/countries/japan (last accessed 19 April 2021). [Google Scholar]
- 21.Blauvelt A, de Bruin‐Weller M, Gooderham Met al. Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389:2287–303. [DOI] [PubMed] [Google Scholar]
- 22.Bosma AL, de Wijs LEM, Hof MHet al. Long‐term effectiveness and safety of treatment with dupilumab in patients with atopic dermatitis: results of the TREAT NL (TREatment of ATopic eczema, the Netherlands) registry. J Am Acad Dermatol 2020; 83:1375–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1 Extensive information on data analysis.
Table S1 Use of concomitant systemic immunosuppressants in the Netherlands (n = 124).
Table S2 Number of individual patients with collected data at specific time points.
Table S3 Expected and available number of individual patients with collected data at specific time points.


