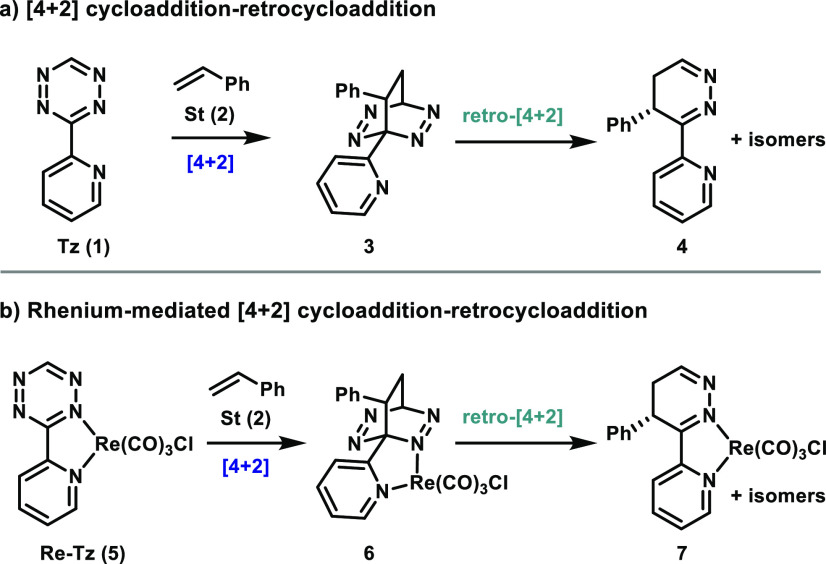
Figure 1.
[4 + 2] Cycloadditions of tetrazine 1 (Tz) and rhenium-coordinated tetrazine 5 (Re-Tz) with styrene (2, St) leads to the formation of intermediates 3 and 6, respectively. This is followed by a retro-[4 + 2] cycloaddition to form dihydropyridazines 4 and 7. The regioisomers in which the Ph group is ortho to the pyridyl substituent are shown. Subsequent oxidation of products 4 and 7 can form pyridazines.