Figure 2.
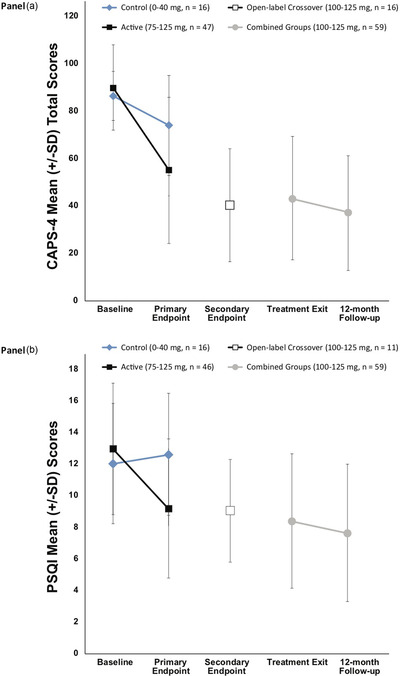
Change over time (A) Pittsburgh Sleep Quality Index (PSQI) and (B) Clinician‐Administered PTSD Scale for DSM‐IV (CAPS‐IV) Total Scores
Note. The primary endpoint occurred 1 month after the second blinded 3,4‐methylenedioxymethamphetamine (MDMA)/placebo session. The blind was broken after the primary endpoint. The active dose groups (100–125 mg) had one additional open‐label MDMA session and completed an assessment 2 months after the third session (i.e., TE). The comparator group (0–40 mg) and the 75–mg group crossed over to receive three open‐label (100–125 mg) sessions, with an assessment 1 month after the second open‐label MDMA session (i.e., secondary endpoint) and again 2 months after the third open‐label MDMA session (i.e., TE). The 12‐month follow‐up visit occurred after the final open‐label MDMA session. Groups were pooled for TE and 12‐month follow‐up endpoints, as all participants had received active doses of MDMA in either the blinded or open‐label crossover segments. PTSD = posttraumatic stress disorder.
