Figure 6.
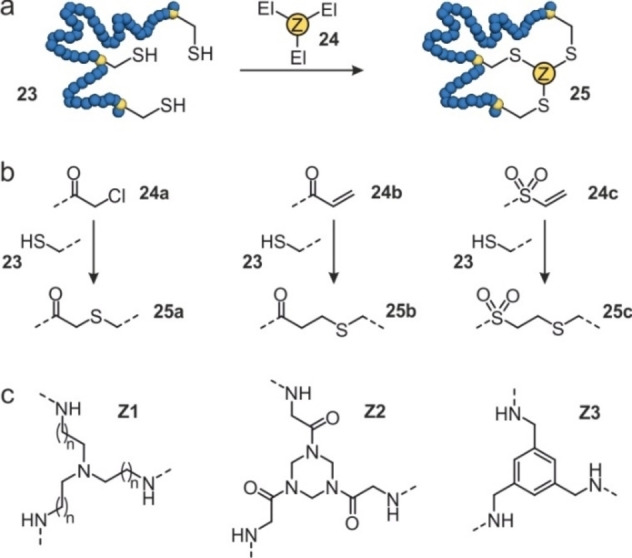
In situ cyclization of proteins (INCYPRO): (a) Schematic overview of protein (blue) with three cysteines (23) that can react with a tris‐electrophile (24) to form a bicyclic protein (25). (b) Chemical structures of the electrophiles attached to the core of the tris‐electrophile: chloroacetamide (24 a), acrylamide (24 b), and vinyl sulfonamide (24 c). They can react with cysteines to form 25 a–c. (c) Chemical structures of the core of the tris‐electrophile based on triethylamine (Z1), triazinane (Z2) and benzene (Z3).
