Abstract
Introduction
Innovative patient engagement models are required to identify people with prodromal and mild Alzheimer's disease who are “hidden” in their communities and not normally found in a memory clinic setting.
Methods
A marketing campaign and a web‐based pre‐screening tool were used to identify individuals at risk of dementia in five European countries. Harmonized clinical evaluation of these patients was performed in participating memory clinics within the MOPEAD project.
Results
A total of 1487 individuals completed the pre‐screening, with 547 of them found to be at risk of dementia (36.8%). Among the subset of 91 patients with a positive pre‐screening result that underwent full clinical evaluation, 49 (53.8%) were diagnosed with either mild cognitive impairment or Alzheimer's disease.
Conclusion
This novel web‐based pre‐screening tool showed to be a valid strategy to identify undiagnosed people with cognitive impairment.
Keywords: Alzheimer's disease, diagnostic gap, early diagnosis, patient engagement, population‐based screening
1. INTRODUCTION
Dementia is a devastating condition with a rapidly increasing prevalence. Projections based on social and demographic trends worldwide suggest that the number of cases could triple in the next 25 years.1 In response, the World Health Organization has made dementia research in management and prevention a global health priority.2
One of the problems we face when fighting dementia is that a large proportion of people with cognitive decline remain undiagnosed or “hidden” in their communities. Although there is limited evidence exploring the impact of timely diagnosis of Alzheimer's disease (AD), some reported benefits include delayed institutionalization, improved patient/carer quality of life, and access to timely counseling and social support.3 However, in most health systems, dementia is underdiagnosed, and diagnosis typically occurs at a relatively late stage in the disease process. Furthermore, it has been hypothesized that one of the reasons for the disappointing results of clinical trials in patients with AD could be the limited effect of these drugs when irreversible neuronal damage has already occurred.4 Therefore, diagnosing patients at early stages of the disease would not only be beneficial for them but could also be crucial in finding new effective treatments. However, the current lack of treatment options to revert the condition, along with the poor knowledge of possible social care interventions, discourages general practitioners’ efforts to reach an early diagnosis5, 6 and dissuades patients from seeking care. The scarcity of patients with early diagnosis impedes the development of effective treatments, and at the same time this lack of treatments makes it difficult to implement screening programs to identify patients in early stages of dementia. To break this loop, a paradigm shift in AD diagnosis is needed, moving toward earlier diagnoses. This shift requires an effort to increase patient engagement and find “hidden” prodromal AD, mild cognitive impairment (MCI), and mild dementia cases.7 The Models of Patient Engagement for Alzheimer's Disease (MOPEAD) project aims to raise awareness of this problem and explores different ways to promote diagnosis at early stages of the disease.8 These mechanisms include the use of (1) innovative internet‐based pre‐screening tools, (2) open‐house initiatives at memory clinics, (3) primary care engagement, and (4) tertiary care engagement through diabetes clinics. This paper describes the methodology and the results of the first strategy, known as RUN1, which comprises an online campaign targeted to individuals between 65 and 85 years of age, who are directed to a web‐based examination to detect people who may have cognitive impairment. The aim of this study was to assess the validity of this method for identifying patients with MCI or AD dementia among the elderly population.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using PubMed. As a paradigm shift toward earlier Alzheimer's disease (AD) diagnosis is advocated, innovative strategies that use web‐based tools to promote early diagnosis of AD are being increasingly used. These relevant citations are appropriately cited.
Interpretation: We present the results of a novel patient engagement model implemented simultaneously in five European countries. We showed that this is a valid and cost‐effective method to identify people with prodromal and mild AD dementia.
Future directions: Our results support the use of web‐based pre‐screening tools to enable timely diagnosis of AD. Furthermore, the MOPEAD project includes three additional innovative patient engagement models: an open‐house initiative at the memory clinics, a primary care‐based patient engagement strategy, and a third strategy based on tertiary care in collaboration with diabetologists. Direct comparison of data from these initiatives will provide more insight into the value of these models.
2. METHODS
2.1. Overall methodology
The internet and social media are tools commonly used to look for medical information.9 This made us consider the use of online marketing campaigns to reach undiagnosed individuals with MCI or AD dementia in numbers much bigger than current mechanisms, like recruitment at health‐care centers. Marketing campaigns were designed to target those individuals meeting the inclusion criteria of this study, taking into account the cultural context of each participating country. When searching for different medical terms related to memory dementia or AD in Google or Facebook, people were redirected to a web (the Webtool) in which they could assess their cognitive status based on two online tests. If the result of this pre‐screening process was positive (impaired performance), the individual was recommended for a visit to a specialized health‐care center to obtain a definite diagnosis. The first 33 participants with a positive result were invited to attend to the Memory Unit for a complete diagnosis procedure.
2.2. Participating centers
Memory clinics from five European countries participated in this study: University Medical Centre in Ljubljana (Slovenia), Fundació ACE in Barcelona (Spain), Karolinska Institutet in Stockholm (Sweden), VU Medical Center in Amsterdam (Netherlands), and University of Cologne Medical Center in Cologne (Germany). The catchment areas of these five centers were targeted for this study. Estimated population aged 65 years and older living within a 50 km radius from these memory clinics included a total of 3.3 million people (143,107 Ljubljana; 879,787 Barcelona; 300,278 Stockholm; 725,308 Amsterdam; and 1,272,367 Cologne).10
2.3. Webtool
We designed a landing page that adapted its appearance to the browser's regional configuration. Individuals were presented with information about cognitive decline and their potential participation in the study in their local languages. Once they agreed to participate in the study, individuals were asked to provide basic demographic data (age, sex, and educational level), and were redirected to a website where they could perform two cognitive tests, the Paired Associates Learning (PAL) and the Spatial Working Memory (SWM) tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB, Cambridge Cognition Ltd., UK). These tests were selected for being relatively short to ensure compliance (4 and 8 minutes, respectively); language‐independent, with translated instructions available for different countries; with accuracy response recordings; and mainly because they are sensitive to visual memory and executive functions, which have been demonstrated to be useful for detecting early AD,11, 12 and highly correlated with neuroimaging13, 14, 15 and cerebrospinal fluid (CSF) AD biomarkers.16
A model designed by Cambridge Cognition Ltd. was used to determine individuals with a positive pre‐screening result (i.e., impaired cognition). The model considered the result obtained in both tests, as well their age, sex, and education. More details on how the model works can be found elsewhere.17
2.4. Inclusion and exclusion criteria
Only individuals between 65 and 85 years of age according to demographic information and no previous diagnosis of cognitive impairment reported were eligible to undergo this pre‐screening.8
2.5. Marketing strategy
People surfing the internet could be the participants themselves, but also a friend or a family member interested in their cognitive assessments. They were directed to the webtool landing page by means of various online marketing techniques. Four alternative strategies were followed in this campaign: (1) Google Adwords: paid ads that appear as results after a dementia‐related Google search, (2) Google display ads: paid banners displayed in dementia‐related sites, (3) Facebook ads: paid advertisements that appear in Facebook directing to the landing page, (4) unpaid: Search engine optimization strategies to achieve top positions in related searches, or project's dissemination activities by partners in websites or social media that link to the webtool landing page.
The initial choice of Google's advertising platform was based on the predominance of Google as a search engine in participating countries. A set of country‐specific dementia‐related keywords was selected based on the reported frequency of these words in Google searches and local investigator criteria (Table S1 in supporting information). It is important to note that the cost per click is determined by market forces (based on bids placed by competing campaigns). Hence it fluctuates with time and varies for different words in different target locations. Given the limited resources, the marketing campaign needed to adapt to these changes to maximize the effect of the investment in each target location during the study period. Changes to the marketing campaign (such as keywords being used, the design or content of the ads/landing pages, or reinforcing the campaign for specific countries) were adopted during the study period if deemed necessary.
2.6. Diagnostic evaluation
A maximum of 33 consecutive patients with a positive pre‐screening per study center was planned to undergo a clinical diagnostic evaluation with a common homogeneous protocol across all sites and countries to ensure the resulting data are comparable. The evaluation has been described elsewhere.8 Briefly it included: (1) physical and neurological examinations; (2) a neuropsychological assessment; (3) functionality evaluation by means of the Clinical Dementia Rating Scale;18 (4) assessment of resource use; (5) affective symptoms evaluation with the Hospital Anxiety and Depression Scale; (6) standard blood workup; (7) optional blood sampling for apolipoprotein E (APOE) haplotype determination; (8) optional CSF with the analysis centralized at the Clinical Neurochemistry Laboratory at Sahlgrenska University Hospital in Gothenburg (Sweden) including measurement of amyloid beta (Aβ) 42, Aβ40, total tau (t‐tau), and phospho‐tau (p‐tau) levels; and (9) neuroimaging evaluation.
2.7. Statistical analyses
We aimed to recruit 100 individuals per country in this initiative, with 33 of them completing the clinical evaluation. We estimated that this sample size allowed us to detect differences of 65% or larger in the positive pre‐screening rates between participating countries in the four pre‐screening initiatives evaluated within the MOPEAD project (power = 80%, two‐sided alpha = 0.01).
The distribution of age groups, sex, and education among study subjects across countries and sources of traffic was compared using chi‐square tests. Mean age was also estimated and compared using analysis of variance (ANOVA) tests. Unconditional logistic regression models were used to explore the following probabilities: completing the pre‐screening process, obtaining a positive pre‐screening result, being evaluated at the memory clinics, and confirming a positive pre‐screening result after clinical diagnostic examination at the memory clinics. To obtain adjusted estimates for the contribution of each factor, the following variables were introduced simultaneously in these models: age groups, sex, education, traffic source, and country of origin.
The positive predictive value (PPV) of the webtool was estimated by dividing the number of confirmed MCI/AD cases after the full clinical evaluation over the total number of individuals with a positive pre‐screening that received a full clinical evaluation. Binomial proportion exact 95% confidence interval for this estimate was obtained.
2.8. Ethics
The webtool was designed taking into account an ethical perspective and protecting data privacy from the very design. Approval from institutional review boards (IRBs) was obtained in all participating countries. IRBs in Germany and the Netherlands required changes to the online platform, which delayed the rollout of the campaign, and, more importantly, forced individuals to go through additional steps to agree to participate in the study.
3. RESULTS
The campaign started in July 2018 in Spain, Sweden, and Slovenia; in October 2018 in Germany and the Netherlands; and ended for all sites in May 2019. During the study period, traffic toward landing pages totaled 72,127 visits (Table 1). Spain and Germany comprised 57% of all this traffic. However, it was in Slovenia where the campaign was most successful, achieving 8110 visits per every 100,000 individuals in the target population (i.e., individuals 65 years or older living within 50 km of the memory clinic). In the remaining countries, estimates ranged from 2740 to 1380 visits per 100,000 individuals from Spain and Germany, respectively. Traffic generated from unpaid sources achieved the best results in Slovenia with two thirds of all traffic in this site. This is mainly explained by the support received from the local Patient´s Association that helped publicize the project in different media. This result contrasted with those from Germany, where almost all traffic was generated through strategies that involved paid advertisement (93.8%).
TABLE 1.
Traffic source and advertising costs by country
| Unpaid | Google Display | Adwords | Total | Visits per 100,000a | Advertising cost | Cost per visit | ||
|---|---|---|---|---|---|---|---|---|
| Country | ||||||||
| Slovenia | 7941 | 317 | 2018 | 1330 | 11606 | 8110 | 2271 € | 0,20 € |
| Spain | 5464 | 12131 | 5776 | 736 | 24107 | 2740 | 5118 € | 0,21 € |
| Sweden | 1840 | 732 | 3365 | 142 | 6079 | 2024 | 2655 € | 0,44 € |
| Netherlands | 1429 | 4781 | 6331 | 229 | 12770 | 1761 | 5882 € | 0,46 € |
| Germany | 1083 | 11977 | 4251 | 254 | 17565 | 1380 | 7638 € | 0,43 € |
| Total | 17899 | 36487 | 21741 | 2691 | 72127 | 2172 | 23565 € | 0,33 € |
Visits per 100,000 individuals aged 65 and above living within 50 km of site.
In total, 23,565 euros were invested to generate traffic to the landing pages of the study centers. This figure results from multiplying the cost of each ad (which varies) by the number of individuals that clicked on the ad who were redirected to the landing pages (i.e., traffic). Of note, throughout the campaign >15 million individuals were exposed to the ads while surfing the web, irrespective of whether they clicked on it or not. The distribution of traffic sources in each site (Table 1) has a great impact on the costs of the campaign, as well as the market price of these ads in the different locations. The distribution of the cost of the different strategies also differed by country (Figure S2 in supporting information). For instance, Facebook ads represented only one third of the cost for campaigns in Spain and Slovenia, more than half for the campaigns in Germany and Sweden, and close to two thirds in the Netherlands. Among Google advertising strategies, displayed ads accounted for one third of the campaign costs in Spain and Germany, <20% in the Netherlands and Slovenia, and only 1.2% in Sweden.
As seen in Figure 1, only a fraction of those individuals visiting the landing pages filled in the demographic data and initiated the cognitive tests (n = 2725, 3.8%). We found that the distribution of age and sex among these patients varied depending on the country of origin and the traffic source. Thus, we found that individuals from Sweden were the oldest (mean age = 71.5, standard deviation [SD] = 5.3), while those from Slovenia were the youngest (on average >2 years younger; mean age = 69.1, SD = 4.7). Also, we found that while females comprised more than two thirds of all participants from Slovenia, in Spain there were slightly more males than females (Table 2). We also observed that Individuals who reached the landing page via Facebook ads tended to be younger, and more frequently of female sex than those recruited via Google display ads, Adwords, or those from unpaid traffic sources (Table S2 in supporting information).
FIGURE 1.
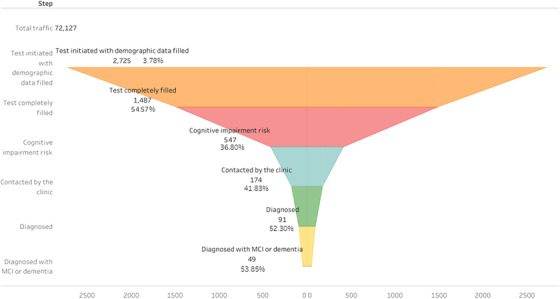
Funnel plot from visits to Alzheimer's disease/mild cognitive impairment (MCI) cases
TABLE 2.
Age, sex, and education according to country of origin
| Slovenia (n = 1018) | Spain (n = 965) | Sweden (n = 223) | Netherlands (n = 429) | Germany (n = 90) | Total (n = 2725) | |
|---|---|---|---|---|---|---|
| Mean age (SD) | 69.1 (4.7) | 70.0 (5.5) | 71.5 (5.3) | 71.1 (5.5) | 69.3 (5.0) | 69.9 (5.2) |
| Age (%) | ||||||
| 65–69 years | 647 (63.6) | 558 (57.8) | 93 (41.7) | 193 (45.0) | 53 (58.9) | 1544 (56.7) |
| 70–74 years | 212 (20.8) | 214 (22.2) | 63 (28.3) | 126 (29.4) | 22 (24.4) | 637 (23.4) |
| 75–79 years | 119 (11.7) | 109 (11.3) | 50 (22.4) | 65 (15.2) | 11 (12.2) | 354 (13.0) |
| 80–85 years | 40 (3.9) | 84 (8.7) | 17 (7.6) | 45 (10.5) | 4 (4.4) | 190 (7.0) |
| Sex (%) | ||||||
| Female | 688 (67.6) | 444 (46.0) | 136 (61.0) | 282 (65.7) | 46 (51.1) | 1596 (58.6) |
| Male | 330 (32.4) | 521 (54.0) | 87 (39.0) | 147 (34.3) | 44 (48.9) | 1129 (41.4) |
| Education (%) | ||||||
| Primary or less | 151 (14.8) | 204 (21.1) | 27 (12.1) | 252 (58.7) | 22 (24.4) | 656 (24.1) |
| Secondary | 507 (49.8) | 320 (33.2) | 68 (30.5) | 110 (25.6) | 27 (30.0) | 1032 (37.9) |
| Undergraduate | 261 (25.6) | 288 (29.8) | 73 (32.7) | 17 (4.0) | 9 (10.0) | 648 (23.8) |
| Postgraduate | 99 (9.7) | 153 (15.9) | 55 (24.7) | 50 (11.7) | 32 (35.6) | 389 (14.3) |
Furthermore, only a little more than half of them (n = 1487) completed the pre‐screening tests. The objective was to have at least 100 patients pre‐screened per country. By the end of August (i.e., month 2), Slovenia had already achieved four times this target. Spain and Sweden hit the 100 target by January, and the Netherlands by February. By the end of the study period, Germany had not reached this target, with only 41 individuals having completed the pre‐screening. Therefore, the proportion of individuals completing the pre‐screening over all traffic varied considerably between countries. For instance, in Germany, these 41 pre‐screened individuals represent only a tiny fraction (0.2%) of all 17,565 visits to the landing page. In contrast, 653 pre‐screened patients in Slovenia represent 5.6% of all visits to the landing page (n = 11,606). The corresponding percentages in Spain (2.2%), Sweden (2.1%), and the Netherlands (1.1%) lie somewhere in between.
When the size of the target population was considered, we found that in Slovenia, the campaign was able to achieve a complete pre‐screening in 456 of every 100,000 individuals in the target population. The corresponding estimates for Spain, Sweden, the Netherlands, and Germany were 60, 42, 19, and 3 pre‐screened patients per 100,000 individuals, respectively.
We were able to explore the factors associated with completing the pre‐screening. As seen in Table 3, compared to those aged below 70 years, older age groups were between 20% and 40% more likely to complete the pre‐screening, while sex and education did not seem to have an effect. Individuals recruited via Facebook were more likely to finalize the pre‐screening, while those via Adwords were less willing to complete the pre‐screening compared to individuals from unpaid traffic sources. Finally, those from Slovenia were the most likely to complete the pre‐screening, while those from the Netherlands were the least likely.
TABLE 3.
Probability of completing the pre‐screening process according to individual characteristics
| Complete (n = 1487) | Incomplete (n = 1238) | |||||
|---|---|---|---|---|---|---|
| N | % | N | % | OR (95% CI) | P | |
| Age | ||||||
| 65–69 years a | 810 | 54.5 | 734 | 59.3 | 1 | |
| 70–74 years | 374 | 25.2 | 263 | 21.2 | 1.40 (1.15–1.71) | <.01 |
| 75–79 years | 205 | 13.8 | 149 | 12.0 | 1.38 (1.08–1.77) | .01 |
| 80–85 years | 98 | 6.6 | 92 | 7.4 | 1.21 (0.88–1.66) | .24 |
| Sex | ||||||
| Femalea | 882 | 59.3 | 714 | 57.7 | 1 | |
| Male | 605 | 40.7 | 524 | 42.3 | 0.97 (0.82–1.14) | .68 |
| Education | ||||||
| Primary or lessa | 297 | 20.0 | 359 | 29.0 | 1 | |
| Secondary | 606 | 40.8 | 426 | 34.4 | 1.22 (0.99–1.52) | .07 |
| Undergraduate | 379 | 25.5 | 269 | 21.7 | 1.16 (0.91–1.48) | .23 |
| Postgraduate | 205 | 13.8 | 184 | 14.9 | 1.11 (0.84–1.45) | .47 |
| Traffic | ||||||
| Unpaid sourcesa | 656 | 44.1 | 410 | 33.1 | 1 | |
| Google Display | 475 | 31.9 | 349 | 28.2 | 1.10 (0.85–1.43) | .46 |
| Adwords | 194 | 13.0 | 354 | 28.6 | 0.60 (0.46–0.79) | <.01 |
| 162 | 10.9 | 125 | 10.1 | 1.39 (1.02–1.90) | .04 | |
| Country | ||||||
| Sloveniaa | 653 | 43.9 | 365 | 29.5 | 1 | |
| Spain | 528 | 35.5 | 437 | 35.3 | 0.68 (0.52–0.88) | <.01 |
| Sweden | 125 | 8.4 | 98 | 7.9 | 0.80 (0.58–1.12) | .20 |
| Netherlands | 140 | 9.4 | 289 | 23.3 | 0.33 (0.24–0.45) | <.01 |
| Germany | 41 | 2.8 | 49 | 4.0 | 0.67 (0.42–1.08) | .10 |
Notes: Odds ratios (OR) and 95% confidence interval (CI) estimates adjusted for all variables in the table using an unconditional logistic regression model.
Reference category.
According to the results of the pre‐screening, a total of 547 individuals were at risk of dementia (36.8%). As seen in Table 4, older age and lower education were the most important predictors of a positive screening. Traffic source and country of origin did not seem to be associated with the screening result. Details on the results of the screening tests have been reported in a previous communication.19
TABLE 4.
Probability of having a positive pre‐screening according to individual characteristics
| Positive (n = 547) | Negative (n = 940) | |||||
|---|---|---|---|---|---|---|
| N | % | N | % | OR (95% CI) | P | |
| Age | ||||||
| 65–69 years a | 274 | 50.1 | 536 | 57.0 | 1 | |
| 70–74 years | 141 | 25.8 | 233 | 24.8 | 1.12 (0.86–1.46) | .39 |
| 75–79 years | 92 | 16.8 | 113 | 12.0 | 1.51 (1.10–2.08) | .01 |
| 80–85 years | 40 | 7.3 | 58 | 6.2 | 1.15 (0.74–1.79) | .55 |
| Sex | ||||||
| Femalea | 310 | 56.7 | 572 | 60.9 | 1 | |
| Male | 237 | 43.3 | 368 | 39.1 | 1.22 (0.97–1.53) | .08 |
| Education | ||||||
| Primary or lessa | 144 | 26.3 | 153 | 16.3 | 1 | |
| Secondary | 227 | 41.5 | 379 | 40.3 | 0.70 (0.52–0.95) | .02 |
| Undergraduate | 116 | 21.2 | 263 | 28.0 | 0.52 (0.37–0.73) | <.01 |
| Postgraduate | 60 | 11.0 | 145 | 15.4 | 0.48 (0.33–0.72) | <.01 |
| Traffic | ||||||
| Unpaid sourcesa | 219 | 40.0 | 437 | 46.5 | 1 | |
| Google Display | 176 | 32.2 | 299 | 31.8 | 1.33 (0.91–1.94) | .14 |
| Adwords | 88 | 16.1 | 106 | 11.3 | 1.40 (0.94–2.10) | .10 |
| 64 | 11.7 | 98 | 10.4 | 0.99 (0.65–1.50) | .95 | |
| Country | ||||||
| Sloveniaa | 225 | 41.1 | 428 | 45.5 | 1 | |
| Spain | 191 | 34.9 | 337 | 35.9 | 0.83 (0.57–1.21) | .33 |
| Sweden | 43 | 7.9 | 82 | 8.7 | 0.82 (0.51–1.31) | .41 |
| Netherlands | 74 | 13.5 | 66 | 7.0 | 1.47 (0.92–2.37) | .11 |
| Germany | 14 | 2.6 | 27 | 2.9 | 0.95 (0.46–1.93) | .88 |
Notes: Odds ratios (OR) and 95% confidence interval (CI) estimates adjusted for all variables in the table using an unconditional logistic regression model.
Reference category.
A subset of all individuals with a positive pre‐screening could be contacted and evaluated in the memory clinic to confirm or discard this result (n = 91, 16.7%). We found that those aged 70 to 74 years and those from Sweden were more likely than others to be evaluated in the memory clinic (Table S3 in supporting information). We also found a high proportion of individuals evaluated in Germany but based only on the eight individuals with a positive pre‐screening result observed there (with six of them being evaluated).
Finally, after this clinical assessment, 49 patients (PPV = 53.8%; 95% confidence interval [CI] = 43.1–64.4) were diagnosed with MCI or AD. Among countries that evaluated >10 individuals this proportion ranged from 71.0% in Spain, to 41.9%% in Slovenia. As seen in Table 5, after adjustment for other factors such as age, sex, or education, those with a positive pre‐screening from Spain and Sweden were more likely to be diagnosed with MCI/AD in the clinical evaluation than patients from other countries. Also, confirmation was higher among those who had been originally recruited via Facebook or Adwords, and lower among those from Google display.
TABLE 5.
Probability of MCI/AD diagnosis after clinical evaluation according to individual characteristics
| MCI/AD (n = 49) | No MCI/AD (n = 42) | |||||
|---|---|---|---|---|---|---|
| n | % | N | % | OR (95% CI) | P | |
| Age | ||||||
| 65–69 years a | 17 | 34.7 | 19 | 45.2 | 1 | |
| 70–74 years | 22 | 44.9 | 14 | 33.3 | 3.33 (0.81–13.66) | .10 |
| 75–79 years | 7 | 14.3 | 8 | 19.0 | 0.56 (0.10–3.20) | .51 |
| 80–85 years | 3 | 6.1 | 1 | 2.4 | 0.83 (0.05–14.98) | .90 |
| Sex | ||||||
| Femalea | 19 | 38.8 | 26 | 61.9 | 1 | |
| Male | 30 | 61.2 | 16 | 38.1 | 2.28 (0.70–7.42) | .17 |
| Education | ||||||
| Primary or lessa | 10 | 20.4 | 8 | 19.0 | 1 | |
| Secondary | 16 | 32.7 | 23 | 54.8 | 0.21 (0.03–1.35) | .10 |
| Undergraduate | 16 | 32.7 | 8 | 19.0 | 1.26 (0.18–8.89) | .82 |
| Postgraduate | 7 | 14.3 | 3 | 7.1 | 1.47 (0.13–17.10) | .76 |
| Traffic | ||||||
| Unpaid sourcesa | 19 | 38.8 | 17 | 40.5 | 1 | |
| Google Display | 16 | 32.7 | 14 | 33.3 | 0.14 (0.02–0.94) | .04 |
| Adwords | 8 | 16.3 | 7 | 16.7 | 11.97 (0.84–169.95) | .07 |
| 6 | 12.2 | 4 | 9.5 | 14.06 (1.67–118.56) | .02 | |
| Country | ||||||
| Sloveniaa | 13 | 26.5 | 18 | 42.9 | 1 | |
| Spain | 22 | 44.9 | 9 | 21.4 | 14.97 (1.87–119.92) | .01 |
| Sweden | 12 | 24.5 | 5 | 11.9 | 14.11 (1.68–118.65) | .01 |
| Netherlands | 2 | 4.1 | 4 | 9.5 | 0.04 (0.00–0.77) | .03 |
| Germany | 0 | 0.0 | 6 | 14.3 | NA | |
Notes: Odds ratios (OR) and 95% confidence interval (CI) estimates adjusted for all variables in the table using an unconditional logistic regression model.
Reference category.
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment.
4. DISCUSSION
The results of this study show that web‐based pre‐screening campaigns targeting the elderly population represent a valid method to identify individuals with MCI or prodromal AD that could possibly have remained undiagnosed or be diagnosed at a later stage. Timely diagnosis is crucial because it grants patients and their families access to counseling and social support, and promotes clinical research aimed at finding a definitive cure for this devastating disease. Our study found that more than one half of those individuals with a positive pre‐screening result were confirmed as MCI/AD in a clinical diagnostic evaluation performed at specialized memory clinics. It is important to note that the validity of this online pre‐screening tool was similar irrespective of age. To put this result into context, we should note that the percentage of individuals diagnosed with MCI/AD among participants of an open‐house initiative performed before the MOPEAD study began in one of the study centers was 37%.20 This result is well below the lower limit of our PPV estimate, even though prevalence among participants on a web‐based tool is expected to be lower due to their younger age.
It is also important to establish to what extent this initiative, which included a marketing campaign and an online pre‐screening tool, was effective in reaching our target population.
Online campaigns, including social media such as Facebook, are increasingly being used to recruit individuals to participate in health studies. Most of these studies specifically target young adults or even adolescents, in areas such as human immunodeficiency virus, pregnancy outcomes, etc.21 However, in instances in which broader populations have been targeted (e.g., smoking cessation trials),22 samples obtained using social media strategies were skewed to the younger demographic groups, reflecting the profile of active social media users. Our initiative was focused explicitly on the elderly population. Because the internet is not so widely used among the elderly, this can affect the effectiveness of the campaign. According to 2016 data from Eurostat, only 45% of those over 65 years of age use the internet at least once a week in Europe, which essentially excludes a large percentage of the target population from getting to know about or to participating in our study.23 Despite this fact, our initiative was able to perform a full pre‐screening in a remarkable 0.5% of individuals aged 65 years and above who live in the area surrounding our study site in Slovenia. Our results in Spain and Sweden were more modest but also entirely satisfactory. Our results in the Netherlands and especially in Germany are somewhat deceptive and seem to be related to the delays in rolling out the study and especially to the additional steps that individuals had to go through in the webtool to participate (which were required by IRBs from these countries). However, in general, our results suggest that these online strategies can be effective in the elderly, despite the limited use of internet. Furthermore, the effectiveness of these strategies is expected to increase as internet use becomes more common in this population. We should keep in mind that internet use in the elderly has risen rapidly in the last years and will likely continue to grow. Note that in the United States, internet use among the elderly went from 22% in 2004 to 67% in 2016.24
Thus, the potential of web‐based tools to identify individuals at risk and promote early diagnosis of MCI/AD is appreciable. In fact, there are several ongoing initiatives, like the Brain Health Registry, an online study recruiting AD patients but also healthy individuals who are interested in neuroscience research. Participating subjects provide their health and lifestyle information by answering a questionnaire and take periodic online brain tests, which are used to identify potential participants for ongoing clinical trials.25 The APT Webstudy is another online registry specifically designed to identify individuals who may be at higher risk for developing dementia among patients aged 50 to 85 years who take online tests every 3 months.26 Finally, the MindCrowd is an online research study in which healthy individuals aged 18 years and older register to take a PAL online test and who might be contacted in a second phase for future memory studies.27
To enhance the dissemination of these initiatives, marketing campaigns that involve advertising costs are used. In our study, the advertising cost per visit to the landing page ranged between 0.20 and 0.46 euros depending on the country and the proportion of paid traffic. Our ability to translate these visits into study subjects that complete the pre‐screening process determines the cost‐effectiveness of the strategy. We were successful in doing so in Spain, Sweden, and especially in Slovenia. However, we were not in Germany and the Netherlands. While the campaigns were relatively successful in attracting peoples’ attention in these countries (in fact, Germany was the second country in number of visits), the additional steps required clearly discouraged participation in the study. Therefore, one of the conclusions of this study is that these initiatives will not be effective when the burden of participation in these online pre‐screening tools is excessive, as reflected by the cost per individual completing the pre‐screening in the Netherlands and especially in Germany. Also, we noted that the Facebook campaign led to higher participation rates than others, and this should be considered for future initiatives. Furthermore, we learned that to be successful these strategies should be able to adapt the pre‐screening tool and web‐based referrals system to the logistics, health policies, and cultural idiosyncrasies of local memory clinics.
Our study has some limitations. Comparing the effectiveness of the campaign in the different countries, our traffic sources, we should keep in mind the dynamic nature of the campaign, with changes mainly driven by costs and performance. Also, internet use among the elderly is not equal across participating countries. Interestingly the countries where the initiative was more successful, Spain and Slovenia, have the lowest estimated use of internet among the elderly (28% and 31%, respectively) which emphasizes the great performance of the campaign in these two countries.23 Along these lines, it is important to note that study participants do not comprise a sample representative of all individuals aged 65 to 85 years for many reasons. Clearly those with memory complaints will be more likely to search for dementia‐related information on the internet and therefore to participate in the study. Furthermore, individuals who regularly use the internet differ in many ways (e.g., age, sex, and education) from those who do not. However, we should point out that our study does not intend to estimate the prevalence of impaired cognition in the general population, but to assess the ability of this webtool to identify individuals at high risk of dementia among study participants. Another limitation is that the platform used to administer the cognitive tests is not available in mobile phones, which represent a large percentage of devices used by the target population. Future initiatives should consider using mobile phone–compatible platforms to avoid this problem. Finally, given the observational nature of our study we cannot assume that all new diagnoses identified during the study are a direct consequence of this initiative, as some individuals could have been diagnosed at some point even if they had not participated in the study.
In summary, the results of the study confirm the validity of this online method to identify MCI and early AD cases. Furthermore, we showed how this initiative was effective in engaging the elderly population, despite the challenge faced by the lower use of the internet among this population. These results confirm the potential of online initiatives to shift the paradigm in AD diagnosis toward earlier diagnosis. Unfortunate situations such as the current COVID‐19 pandemic underscore the importance of developing valid online tools to achieve these types of health‐care goals. Future direct comparison with other innovative patient‐engaging strategies within MOPEAD (including an open‐house initiative in the same participating memory clinics, a primary care–based strategy, and a tertiary care–based strategy through diabetes clinics) will allow us to contextualize these results and determine whether one strategy should be favored over the others.
CONFLICT OF INTEREST
Laura Campo is a full‐time employee of Eli Lilly Italia S.p.A. and shareholder of Eli Lilly.
MEMBERS OF MOPEAD CONSORTIUM
Sergi Valero, Isabel Rodriguez, Miren Gurruchaga, Fatima Iradier, Mark Belger, Annette Dumas, Craig Shering, Peggy Maguire, Kristin Semancik, Inmaculada Pérez Garro, Gunilla Johansson, Anders Wimo, Stefania Zlobec, David Krivec, Lena Sannemann, Jaka Bon, Rafael Simo, Andreea Cuidin, Marissa Zwan, Lisa Vermunt, Malou Stoekenbroek, Lisa Waterink, Jean Georges.
Supporting information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement No 115985. This Joint Undertaking receives support from the European Union's Horizon 2020 Research and Innovation program and the European Federation of Pharmaceutical Industries and Associations. www.imi.europa.eu/.
Rodrigo A, Trigueros P, Jamilis L, et al. Identification of undiagnosed dementia cases using a web‐based pre‐screening tool: The MOPEAD project. Alzheimer's Dement. 2021;17:1307–1316. 10.1002/alz.12297
REFERENCES
- 1.Prince M, Wimo A, Guerchet M ADI. World Alzheimer report 2015: the global impact of Dementia | Alzheimer's disease international. World Alzheimer Rep. 2015:1‐87. [Google Scholar]
- 2.Wortmann M. Dementia: a global health priority ‐ Highlights from an ADI and World Health Organization report. Alzheimer's Res Ther. 2012;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois B, Padovani A, Scheltens P, Rossi A, Dell'Agnello G. Timely diagnosis for Alzheimer's disease: a literature review on benefits and challenges. J Alzheimers Dis. 2016;49:617‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling R. A., Jack C. R. Jr, Aisen P. S. (2011) Testing the right target and right drug at the right stage. Sci Transl Med., 3(111), 111cm33. 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernooij‐Dassen MJFJ, Moniz‐Cook ED, Woods RT, et al. Factors affecting timely recognition and diagnosis of dementia across Europe: from awareness to stigma. Int J Geriatr Psychiatry. 2005;20:377‐386. [DOI] [PubMed] [Google Scholar]
- 6.Moore V, Cahill S. Diagnosis and disclosure of dementia–a comparative qualitative study of Irish and Swedish General Practitioners. Aging Ment Health. 2013;17:77‐84. [DOI] [PubMed] [Google Scholar]
- 7.Boada M, Santos‐Santos MA, Rodríguez‐Gómez O, et al. Patient engagement: the fundació ACE framework for improving recruitment and retention in Alzheimer's disease research. J Alzheimer's Dis. 2018;62:1079‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez‐Gómez O, Rodrigo A, Iradier F, et al. The MOPEAD project: advancing patient engagement for the detection of “hidden” undiagnosed cases of Alzheimer's disease in the community. Alzheimer's Dement. 2019. [DOI] [PubMed] [Google Scholar]
- 9.Fiksdal AS, Kumbamu A, Jadhav AS, et al. Evaluating the process of online health information searching: a qualitative approach to exploring consumer perspectives. J Med Internet Res. 2014;16:e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NASA SEDAC Population Estimator. n.d. https://sedac.ciesin.columbia.edu/mapping/popest/pes-v3/ (accessed October 21, 2019).
- 11.Sahakian BJ, Morris RG, Evenden JL, et al. A comparative study of visuospatial memory and learning in Alzheimer‐type dementia and parkinson's disease. Brain. 1988;111:695‐718. [DOI] [PubMed] [Google Scholar]
- 12.Swainson R, Hodges JR, Galton CJ, et al. Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord nd;12:265‐280. [DOI] [PubMed] [Google Scholar]
- 13.Meyer P, Feldkamp H, Hoppstädter M, King AV, Frölich L, Wessa M, Flor H. (2013) Using voxel‐based morphometry to examine the relationship between regional brain volumes and memory performance in amnestic mild cognitive impairment. Front Behav Neurosci. 7:89. 10.3389/fnbeh.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Rover M, Pironti VA, McCabe JA, et al. Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia. 2011;49:2060‐2070. [DOI] [PubMed] [Google Scholar]
- 15.Nathan PJ, Lim YY, Abbott R, et al. Association between CSF biomarkers, hippocampal volume and cognitive function in patients with amnestic mild cognitive impairment (MCI). Neurobiol Aging. 2017;53:1‐10. [DOI] [PubMed] [Google Scholar]
- 16.Nathan PJ, Galluzzi S, Marizzoni M, et al. Characterization of cognitive function with the cantab in individuals with amnestic mild cognitive impairment in relation to hippocampal volume, amyloid, and tau status: preliminary baseline results from the PharmaCog/european‐ADNI study. Alzheimer's Dement. 2015;11:P564. [Google Scholar]
- 17.Abbott RA, Skirrow C, Jokisch M, et al. Normative data from linear and nonlinear quantile regression in CANTAB: cognition in mid‐to‐late life in an epidemiological sample. Alzheimer's Dement (Amsterdam, Netherlands). 2019;11:36‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 2012;43:2412‐2412. [DOI] [PubMed] [Google Scholar]
- 19.Dente P, Baker E, Cormack FK, et al. P4‐603: interim analysis of online screening as a recruitment strategy for the models of patient engagement in Alzheimer's disease (mopead) initiative. Alzheimer's Dement. 2019;15:P1555‐1556. [Google Scholar]
- 20.Rodríguez‐Gómez O, Abdelnour C, Jessen F, Valero S, Boada M. Influence of sampling and recruitment methods in studies of subjective cognitive decline. J Alzheimer's Dis. 2015;48:S99‐107. [DOI] [PubMed] [Google Scholar]
- 21.Thornton L, Batterham PJ, Fassnacht DB, Kay‐Lambkin F, Calear AL, Hunt S. Recruiting for health, medical or psychosocial research using Facebook: systematic review. Internet Interv. 2016;4:72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frandsen M, Walters J, Ferguson SG. Exploring the viability of using online social media advertising as a recruitment method for smoking cessation clinical trials. Nicotine Tob Res. 2014;16:247‐251. [DOI] [PubMed] [Google Scholar]
- 23.Eurostat. n.d. A look at the lives of the elderly in the EU today. https://ec.europa.eu/eurostat/cache/infographs/elderly/index.html (accessed October 21, 2019).
- 24.Hunsaker A, Hargittai E. A review of Internet use among older adults. New Media Soc. 2018;20:3937‐3954. [Google Scholar]
- 25.Brain Health Registry. n.d. https://www.nia.nih.gov/alzheimers/clinical-trials/brain-health-registry (accessed November 1, 2019).
- 26.Alzheimer Prevention Trials (APT) Webstudy. n.d. https://www.nia.nih.gov/alzheimers/clinical-trials/alzheimer-prevention-trials-apt-webstudy (accessed November 1, 2019).
- 27.MindCrowd. n.d. https://www.nia.nih.gov/alzheimers/clinical-trials/mindcrowd (accessed November 1, 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information


