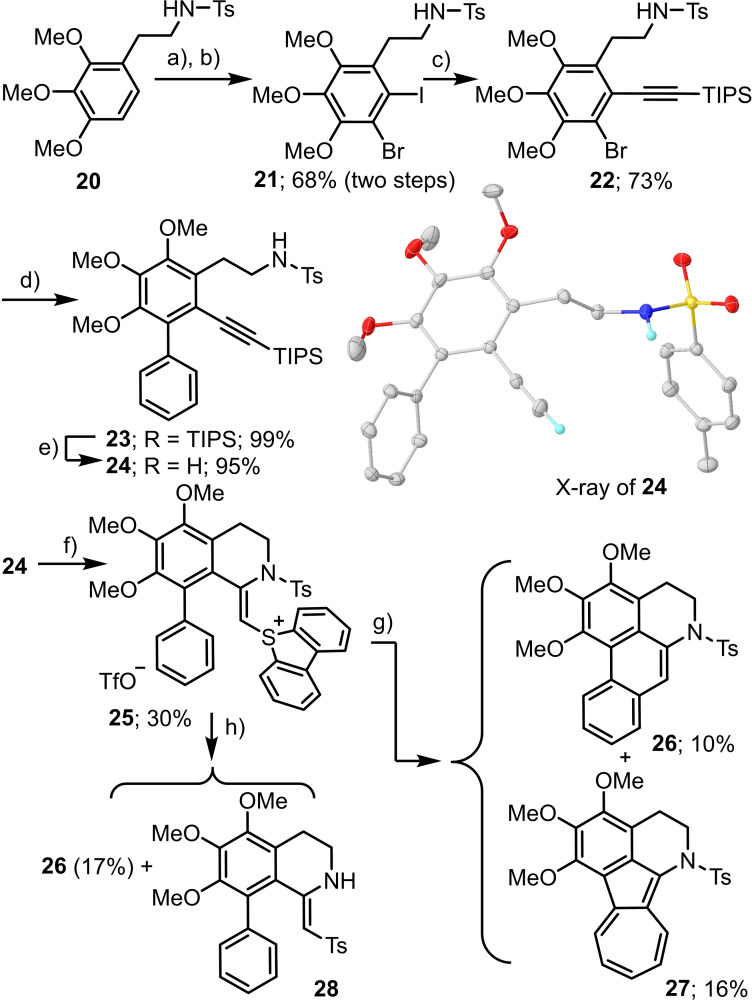
Scheme 7.
Studies towards the synthesis of the 6a,7‐dihydroaporphine scaffold; a) NBS, 60 °C, 16 h; b) NIS, TFA, CH3CN, μW 110 °C, 16 h.; c) TIPS‐acetylene, Pd(PPh3)2Cl2, CuI, NEt3, THF, μW 90 °C, 60 h.; d) PhB(OH)2, Pd(PPh3)2Cl2, Na2CO3, DME/H2O, μW 120 °C, 26 h.; e) TBAF, THF, rt, 2 h.; f) dibenzothiophene‐S‐oxide, Tf2O, CH2Cl2, −50 °C→−20 °C, 16 h.; g) [Ru(bipy)3]2 ⋅ 6H2O (3 mol%), blue LEDs, Cs2CO3 (1.4 equiv.), CH3CN; h) KH (3.0 equiv.), KOt‐Bu (10 mol%). X‐ray of 24 drawn at 50 % probability level; only amide and ethynyl protons shown.[15]