SCHEME 1.
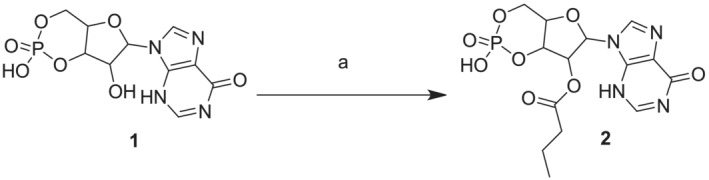
Reagents and conditions: Butyric anhydride, triethylamine, anhydrous pyridine, rt, 48 h. all reactions were followed by TLC carried out on Merk silica gel 60 F254 plates with a fluorescent indicator on the plates were visualized with UV light (254 nm). Preparative chromatographic purifications were performed using a silica gel column (Kieselgel 60). Solutions were concentrated with a Buchi R‐114 rotary evaporator at low pressure. Melting points, determined using a Buchi melting point B‐540 instrument, are uncorrected and represent values obtained on chromatographically purified material. RP‐HPLC preparative purification was performed on a Shimadzu prominence LC‐20 AP system equipped with a multiwavelength prominence SPD‐20A UV–vis detector on a Phenomenex Kinetex XB‐C18 column (5 mm, 21.2 × 250 mm) employing the following solvents: A: 100% acetonitrile in 0.1% TFA, B: 100% H2O in 0.1% TFA. The operational conditions were as follows: Linear‐gradient of 5%–70% acetonitrile + 0.1% TFA in 30 min, using a flow rate of 30 ml·min‐1. The purity of the products was assessed by analytical RP‐HPLC using a Phenomenex Kinetex XB‐C18 column (5 μm, 4.6 × 250 mm). The column was connected to a Rheodyne model 7725 injector, a Shimadzu‐10 ADsp HPLC system, a Shimadzu SPD‐20 a/SPD‐20 AV UV–vis detector set to 254 nm. Mass spectra were performed on LTQ Orbitrap XL™ Fourier transform mass spectrometer (FTMS) equipped with an ESI ION MAX™ source (Thermo fisher, San José, USA)—Negative mode. 1H‐NMR spectrum was recorded on Varian mercury plus 400 MHz instrument. Chemical shifts are reported in parts per million. The following abbreviations are used to describe peak patterns when appropriate: S (singlet), d (doublet), t (triplet), m (multiplet), bs (broad singlet)
