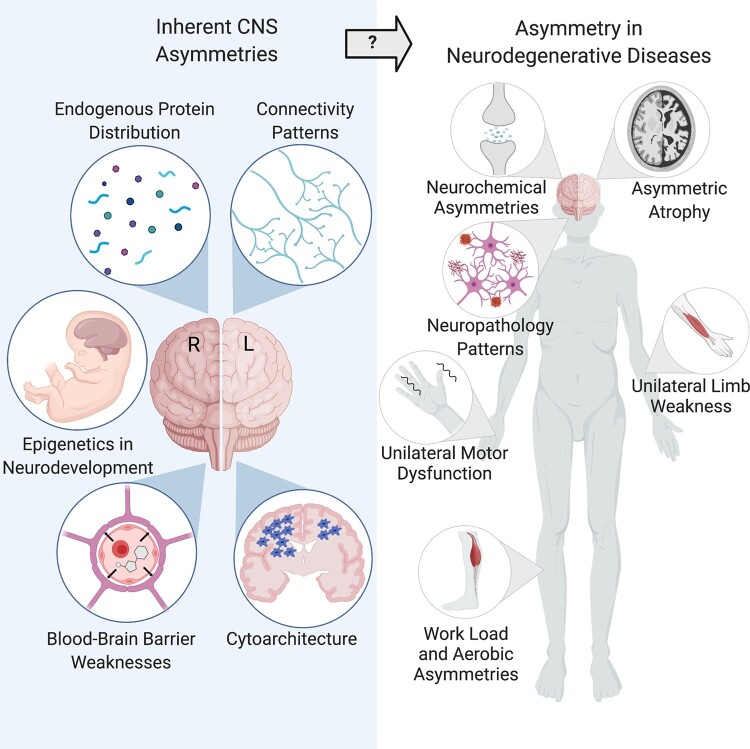
Abstract
The lateralization of the human brain may provide clues into the pathogenesis and progression of neurodegenerative diseases. Though differing in their presentation and underlying pathologies, neurodegenerative diseases are all devastating and share an intriguing theme of asymmetrical pathology and clinical symptoms. Parkinson’s disease, with its distinctive onset of motor symptoms on one side of the body, stands out in this regard, but a review of the literature reveals asymmetries in several other neurodegenerative diseases. Here, we review the lateralization of the structure and function of the healthy human brain and the common genetic and epigenetic patterns contributing to the development of asymmetry in health and disease. We specifically examine the role of asymmetry in Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis, and interrogate whether these imbalances may reveal meaningful clues about the origins of these diseases. We also propose several hypotheses for how lateralization may contribute to the distinctive and enigmatic features of asymmetry in neurodegenerative diseases, suggesting a role for asymmetry in the choroid plexus, neurochemistry, protein distribution, brain connectivity and the vagus nerve. Finally, we suggest how future studies may reveal novel insights into these diseases through the lens of asymmetry.
Keywords: brain lateralization, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, multiple sclerosis
Lubben et al. review the lateralization of the CNS in the context of the common asymmetric phenotypes among neurodegenerative diseases. This evidence suggests that lateralization may confer vulnerability to one brain hemisphere, providing clues to the pathogeneses and progression of these neurological diseases, which merit further research.
Graphical Abstract
Graphical Abstract.
Introduction
Brain asymmetry and lateralization have been investigated for over a century. Broca and Wernike’s findings of language lateralization1,2 and Sperry’s split-brain experiments3 contributed to the early understanding of brain laterality and laid the foundation of understanding brain asymmetry. Today, it is well-established that in the majority of people, the left-brain hemisphere is dominant for language processing and speech, whereas the right hemisphere is dominant for visuospatial functions.4,5 Lateralization of the brain is also clearly seen in motor function with the typical preference for one side of the body over the other, most obviously seen in ‘handedness’. Despite increased investigation into brain asymmetry and its development, much of the functions and consequences of this fundamental lateralization of the CNS remain largely unknown.
Hemispheric asymmetry is a feature of brain organization across many species, including both invertebrates and vertebrates.6,7 The development of brain laterality likely provides some degree of evolutionary advantage, as it can be seen throughout the animal kingdom and is associated with increased cognitive ability.8,9 One hypothesis suggests that lateralization of the brain allows for more efficient function of the hemispheres and prevents feedback from conflicting CNS responses from occurring simultaneously.7,10–12 However, does this complex delegation of tasks to particular hemispheres come with a cost of increased hemispheric vulnerability to brain pathologies like neurodegenerative disease? If so, how and why?
Clinical neuroscience has long recognized hemispheric asymmetries found across many psychiatric, neurological and neurodevelopmental disorders, which has been recently summarized in depth.13 The ENIGMA (Enhancing Neuro-Imaging Genetics through Meta-Analysis) consortium laterality working group (http://enigma.ini.usc.edu/ongoing/enigma-lateralization-working-group/. last accessed September 13, 2021) has laid a foundation for exploring the connection between structural asymmetry in the human brain and neurological disorders, mapping structural asymmetries in healthy individuals as well as in obsessive–compulsive disorder, major depressive disorder and autism spectrum disorder.14 Similarly, abnormal brain lateralization has been studied in schizophrenia, which has been previously reviewed.15–21 The most common neurological disorder, stroke, also typically affects one side of the brain and thus symptoms and recovery may be influenced by hemispheric asymmetries.22–26 Though this lateralization in neurological disease is well-documented, much research is needed to explore how these asymmetries may connect with pathogenesis and progression, particularly among neurodegenerative diseases.
This review will focus on four common neurodegenerative diseases in which asymmetric features are prominent but often neglected in research. The neurodegenerative diseases of Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS) all have asymmetric pathological features. Unilateral symptom onset distinguishes Parkinson’s disease in the clinic27; likewise, ALS and MS often include an onset of limb weakness or numbness on only one side of the body.28,29 Alzheimer’s disease, while lacking the obvious clinical asymmetries, also is characterized by asymmetric neurodegeneration and perhaps vulnerability of the left hemisphere.30–32 Given that the pathogeneses of all these neurodegenerative disorders remain enigmatic, we propose that understanding the fundamental asymmetries in the CNS could provide clues into the onset and/or spread of these diseases, which may guide new research avenues for biomarkers or treatment strategies. Such research is essential as the global burden of these diseases continues to grow while limited to no disease-modifying treatment options exist. Alzheimer’s disease is the fifth leading cause of death in the U.S. in people age 65 and older and affects almost 44 million people globally.33 Parkinson’s disease affects around 1% of people over the age of 65 in the U.S.34,35 As the ageing population expands, these numbers are expected to increase dramatically.33,34 Likewise, ALS and MS, while comparatively less common, represent a significant health burden, with the latter also impacting young people, and case numbers have also been on the rise.36,37 Despite years of dedicated research, these diseases are still not well understood, suggesting that new avenues of research may be needed.
We propose two broad hypotheses of why an asymmetric component characterizes these neurodegenerative diseases. The first, and the focus of this review, suggests that the brain develops with inherent differences in hemispheres which makes one side more vulnerable such that the initiation of pathology will first precipitate the disease in only one hemisphere. Alternatively, the brain could develop with the same relative vulnerability of the hemispheres, but after the initiation of pathology on both sides, some unknown factor or factors lead to degeneration at a more rapid pace on one side. Given the clinical variability within each neurodegenerative disease, both possibilities are worth considering and a combination of processes may be at play.
In this review, we give a brief overview of the inherent asymmetry in the healthy human brain. We discuss the clinical asymmetries associated with neurodegenerative diseases and their relevance to disease severity and progression, while also comparing asymmetry in early and late disease progression and patterns of neuropathology. Finally, we consider possible mechanisms leading to these asymmetries and suggest how future research may reveal novel insights into these diseases through the lens of asymmetry.
The healthy asymmetric brain
Asymmetry in structure and connectivity
Several structural and functional asymmetries are present in the human brain and appear early in development. Recent work by the ENIGMA consortium has thoroughly mapped brain anatomical asymmetry in a large cohort of healthy individuals from across the globe. Their study of cortical asymmetry found that the left hemisphere exhibits greater cortical thickness in the anterior cortex, while rightward asymmetry exists in posterior cortex, creating a global ‘torque’ pattern that has been widely reported.38 This study, as well as others, have also found specific regions (the transverse temporal gyri, opercular part of the inferior frontal gyrus, and planum temporale) to be larger on the left hemisphere, which is linked to a functional asymmetry in language processing.39–41 However, this lateralization is complex, with a few language-related subregions showing greater surface area in the right hemisphere.41 The bilaterally paired subcortical structures also are asymmetric by volume; in particular, the thalamus, putamen and pallidum are larger in the left hemisphere, and the hippocampus, amygdala, nucleus accumbens and caudate nucleus are larger in the right.42 Asymmetry in the epithalamus is also conserved across vertebrate species, though it is more subtle in mammals.43 This region contains the bilaterally paired habenular nuclei which are involved in dopaminergic circuits (governing movement and reward behaviours), spatial learning and attention, conditional avoidance response, and regulation of circadian rhythms and aspects of sleep.44 Interestingly, the habenular nuclei exhibit left–right differences in size, cell-type composition, distribution of neurotransmitters and connectivity.44 There are no sex differences in total mean cortical thickness or in key language regions. However, on a regional level, there is a leftward asymmetry in males in the parahippocampal gyrus and rightward asymmetry in the entorhinal cortex in females; these regions are relevant to Alzheimer’s disease, which disproportionately affects women.45–47 Leftward asymmetry in cortical thickness and in the putamen also varies with age, which also may be relevant for neurodegenerative diseases that typically occur later in life.42,48
Further functional asymmetries have been shown in the dispersal of neurites—axonal and dendritic projections—in the healthy brain. Such asymmetries have previously been reported in relatively small, post-mortem histological studies49–51; however, recent work on two large separate cohorts of healthy participants was conducted using neurite orientation dispersion and density imaging. This work found asymmetric neurite orientation dispersion, a tortuosity measure, towards frontal areas and early auditory areas in the left and right hemispheres, respectively.52 In addition, estimations of neurite density found greater leftward density in the early auditory, inferior parietal and temporal–parietal–occipital areas.52 These findings are in line with the sum of histological studies and suggest microstructural asymmetries in grey matter. In addition to left–right differences in grey matter volume, diffusion tensor imaging has also revealed a broad leftward asymmetry in the microstructure and connectivity of white matter. Several functional MRI studies indicate greater functional connectivity in the right hemisphere, but the direction of this asymmetry seems to shift at various stages of development. Some suggest a pattern of lateralization in which the left hemisphere has greater inter-connectivity, while the right hemisphere interacts across both hemispheres, allowing for visuospatial and attention processing.9,18 The global torque pattern of the brain is also observable in white matter tracts.53
One of the most apparent asymmetries in humans is the phenomenon that humans tend to prefer one side of the body over the other. The most obvious and well-studied preference is that of handedness, though humans also show subtle preferences for one foot over the other and even for head turning direction.54–57 Definitions of ‘handedness’ can vary, but it is typically defined by the preferred hand for writing. The majority of humans exhibit right handedness, with only about 10.6% of the population being left-handed.58 Men are also slightly more likely to be left-handed than women.59 Cultural factors may impact hand preference (for example, a bias against left handers may cause some children to act right-handed despite their initial, natural preference),60 but there does appear to be a neurological basis for this phenomenon. Asymmetry in functional activation of the motor cortex reflects handedness, where greater activation occurs on side contralateral to the hand movement.61,62 Hemispheric differences in the microstructure of the corticospinal tract also predict the degree of functional dexterity in the right hand compared to the left.63 Some studies have shown that left-handed individuals generally exhibit less asymmetry, suggesting that left hand dominance may reflect a lack of asymmetrical development more than an opposite structure.62,64,65 Left handedness is also associated with greater functional connectivity between hemispheres in language networks.66 However, recent studies on large cohorts found no correlations between handedness and anatomical asymmetries, 14,67,68 although one looking at global asymmetrical organization did find patterns of abnormal skew associated with left-handed individuals.53
The development of the asymmetric brain
Exactly how and when brain lateralization develops is still being unraveled. Thus far it has been shown that a combination of genetic and environmental factors can impact the lateralized development of the brain, which has previously been reviewed in depth.8 Structural asymmetries as well as handedness can be observed even during early development in humans, suggesting that fundamental genetic pathways establish lateralization.39,40,65,69–74 Left–right body asymmetry is established during the early stages of development in part through Nodal signalling, which relies on ciliary movement; indeed, gene mutations leading to ciliary defects often results in the condition of situs inversus, in which organ placement is reversed.8,75 People with this rare condition do not show an increased prevalence of left-handedness and retain language dominance in the left hemisphere, suggesting that the processes that establish brain lateralization and handedness are different than those that control the asymmetrical organization of visceral organs.76–78 The work of the ENIGMA consortium has found low but significant heritability for asymmetry in several cortical and subcortical regions.41,42 Another study found heritability of 4–13% for the more global asymmetrical skew pattern of the brain.53 Likewise, handedness is a weak genetic trait that has a heritability estimate of about 0.25 in twin studies. SNP-based heritability estimates are lower, between 1.2 and 6%.66,79,80 No single gene has been found to control for handedness or other forms of lateralization in the human brain; rather, the consensus is that these complex phenotypes are controlled by a variety of genetic loci and possibly environmental influences.8
Recent genome-wide association studies (GWAS) on large datasets have begun to reveal loci that are associated with brain lateralization. From these studies, cilia and cytoskeleton-related pathways have emerged as common contributors to brain lateralization, as well as some loci involved in neuronal development and organization.66,75,79,81 Many of these associated genes are those which show higher mRNA expression at early to mid-prenatal stages of development.81 Looking at more broad measures of brain asymmetrical skew, however, did not reveal any significant GWAS loci.53 Future region-specific studies are needed to further pinpoint genes and mechanisms which contribute to brain lateralization.
Given that only a fraction of brain structural asymmetry and handedness can be explained by heritability, it is likely that non-shared environmental factors and epigenetics play a major role in determining lateralization. Likewise, since the two brain hemispheres are genetically identical, at least in terms of DNA sequences, genomic differences between the hemispheres most likely originate in epigenetics and manifest as different chromatin structure and function. 98% of our genome is not protein-coding (instead designated as intronic or intergenic) and encodes various gene regulatory elements. It is at these regions that hemispheric differences likely exist. Epigenetic regulation includes DNA methylation and post-translational histone modifications, which influence gene transcription, as well as post-transcriptional regulation by small non-coding RNAs. These marks have been shown to influence neurodevelopment,82–85 and it is likely that these epigenetic modifications help explain complex phenotypes that have no clear primary genetic heritability.86 Even in monozygotic twins, several germline mutations, which occurred after twinning, were evident, including methylated CpG to TpG transitions.87
Environmental factors during foetal development have been shown to influence both motor and language lateralization; these factors include season of birth, foetal posture and hormone exposure.7,86 A more recent study found that the probability of left-handedness is associated with a number of environmental factors, including year and country of birth, season of birth, birthweight, and sex, but these factors were not able to reliably predict handedness.88 Maternal prenatal stress has also been associated with the development of brain laterality; in animal models, psychological stress and traumatic life events during gestation led to high rates of mixed handedness as well as asymmetry of dopaminergic activity in offspring.6,89 It is likely that epigenetic mechanisms mediate these environmental effects on the establishment of handedness.6 Stress during development is known to modulate DNA methylation.90,91 While the effects of this methylation could be broad, one study illustrates an example of its relevance to lateralization: methylation levels in the promoter region of LRRTM1 was associated with atypical handedness, and this gene has previously been linked to handedness as well as neurodevelopmental disorders.8,75
The development of asymmetry in avian species also provides support for epigenetic regulation of lateralization. Both chickens and pigeons have demonstrated asymmetry in head turning behaviour and visual and spatial skills; several studies have demonstrated a profound effect of light and posture on these asymmetries, mediated by epigenetic factors in the final stages of incubation, which has been previously reviewed in depth.6–8,92 Such light-induced mechanisms may contribute to similar associations between handedness and season of birth in humans, but much more research is needed.6
In humans, epigenetic asymmetries have been found in spinal tissue during development, which likely play a role in establishing handedness. mRNA expression has a prominent asymmetrical pattern in the C2 to T2 segments of the spinal cord, which includes the segments that innervate the hands. This asymmetry featured increased gene expression on the right spinal cord, which peaked at 8 weeks post-conception; this finding aligned with increased DNA methylation (which typically silences genes) on the left side. Differences in miRNA expression, another mode of epigenetic regulation, were also found, particularly in the TGF-β signalling pathway, which has previously been linked to handedness. This suggests that while handedness and brain structural asymmetries likely share some ontogenetic pathways in the brain, these phenotypes develop by different mechanisms to some extent. This aligns with the inconsistency of association between brain anatomical asymmetries and handedness.41,42,53 Thus, when making connections between handedness and brain lateralization, researchers must take care to avoid overgeneralizations.
The Labrie lab investigated whether epigenetic differences are present between the brain hemispheres of healthy individuals and found hemispheric differences to be prevalent in the neuronal epigenomes of the human prefrontal cortex. Specifically, we found that the left hemispheres of healthy individuals had a greater level of methylation and that these differences were attributed primarily to CpH methylation, an epigenetic mark of repression of neuronal promoters and enhancers.93 Interestingly, in our study, the CpH methylation specifically affected genes involved with neurodevelopment and synaptic organization. Since this study was conducted in older adults, it is unknown if these differences reflect those present at birth, and methylation does change with age94; nevertheless, these methylation asymmetries represent a significant asymmetry that may influence brain lateralization throughout life and could have broader consequences.
Asymmetry in epigenetic regulation should manifest in differences in gene expression between brain hemispheres. Indeed, asymmetries in gene expression have been found in in adult cortex (specifically superior temporal and primary auditory cortex) in gene pathways related to neurcircuitry, namely synaptic transmission, nervous system development and glutamate receptor activity.95 Transcriptomic asymmetries have also been found in human embryos and foetuses.80,96,97 In contrast, other studies have found global symmetry in gene expression during development, but these were conducted in small sample sizes.98,99 This adds further support to an epigenetic origin of lateralization early in development, though more research is needed.
All the structural and functional asymmetries that develop early in the CNS in healthy individuals could contribute to greater vulnerabilities in one hemisphere, which could help explain the asymmetric clinical presentation seen in many neurodegenerative diseases.
Asymmetry in neurodegenerative diseases
Parkinson’s disease
Parkinson’s disease is a progressive neurodegenerative disease characterized by misfolded protein aggregates of alpha-synuclein (α-syn) and the loss of dopaminergic neurons in the substantia nigra (SN). Parkinson’s disease is associated with both nonmotor and motor symptoms, including tremors, rigidity and bradykinesia. Parkinson’s disease is differentiated from other parkinsonian conditions by a unilateral symptom onset, where one side of the body begins to experience motor symptoms first.27 Even in the prodromal stage of the disease, distinct asymmetry in arm swing can be found, which may be used for earlier detection and diagnosis.100 Despite the disease symptoms becoming bilateral with disease progression, the initially afflicted side remains worse throughout the course of the disease.101 In Parkinson’s disease patients, it has been shown that the side of motor symptom onset corresponds with dopaminergic neuronal loss in the contralateral SN.102 Parkinson’s disease is characterized by misfolded α-syn aggregates into intracellular inclusions known as Lewy bodies as well as insoluble fibrils found mostly near axons (Lewy neurites). These pathologic aggregates follow a stereotypical spread originating in the brainstem in the dorsal motor nucleus of the vagus nerve and the olfactory bulb and eventually spreading to the prefrontal cortex in late stages of the disease.103 Lewy body pathology is typically only quantified in one hemisphere at autopsy; however, a recent Parkinson’s disease case study found that some patients exhibited more α-syn pathology in the hemisphere corresponding to motor symptom dominance104; more studies are needed to further delineate this phenomenon.
This hallmark asymmetry is not exclusive to motor symptoms. In fact, asymmetric differences in psychotic symptoms and cognitive impairment, such as poor verbal fluency, object naming, and verbal memory, have been seen to predominate in those with right side motor symptoms whereas deficiencies in prosodic emotion recognition have been associated with left side motor symptoms.105,106 Interestingly, the degree of asymmetry and side of symptom onset are associated with differing rate of disease progression, severity of symptoms and disease duration93,107–109; thus, side of symptom onset provides relevant information for disease prognosis. Despite this unilateral asymmetry being well documented, its origin and role in disease pathology is still not well understood (Table 1).
Table 1.
Summary of asymmetry in neurodegenerative disease
| Disease | Key characteristics | Asymmetric pathology | Asymmetric clinical symptoms |
|---|---|---|---|
| Parkinson’s disease |
Loss of dopaminergic neurons in the substantia nigra α-syn aggregates (Lewy bodies and neurites) Motor and non-motor symptoms including tremor, bradykinesia, and constipation |
Loss of dopaminergic neurons contralateral to motor symptom onset Asymmetric distribution of α-syn aggregates |
Unilateral motor symptom onset Asymmetry in arm swing, often before clinical diagnosis Cognitive symptoms corresponding to side of symptom onset |
| Alzheimer’s disease |
Loss of neurons accompanied by memory loss and other cognitive deficits Amyloid-beta plaques and Tau tangle pathology |
Grey matter atrophy occurs earlier and progresses faster in left hemisphere compared to right Greater loss of neuronal connections in the left hemisphere Asymmetric reductions in glucose metabolism Accelerated asymmetrical cortical thinning, resulting in a loss of normal asymmetry Asymmetric distribution of amyloid-beta pathology |
Poor verbal memory and language impairment or visuospatial deficits correlated with side of greater neurodegeneration and amyloid-beta pathology |
| Amyotrophic lateral sclerosis (ALS) |
Loss of upper and lower motor neurons, resulting in motor dysfunction and ultimately paralysis Aggregation of ubiquinated proteins in motor neurons, especially TDP-43 |
Grey matter loss corresponding to side of symptom onset Asymmetries in TDP-43 distribution have not been studied in ALS, but have been found in frontotemporal dementia and primary progressive aphasia |
Unilateral limb weakness at onset, typically in the dominant limb Motor symptoms remain worse on side of onset as symptoms progress |
| Multiple sclerosis (MS) |
Progressive demyelinating disease resulting in varied symptoms including limb weakness, sensory disturbances, and bowel dysfunction Likely an auto-immune disorder |
Aerobic performance and metabolic asymmetries between limbs Inflammation of the optic nerve in one eye Asymmetric distribution of lesions early in disease Asymmetric grey matter loss in the left hemisphere |
Unilateral limb weakness at onset in some but not all cases |
Alzheimer’s disease
Alzheimer’s disease is a fatal, progressive neurodegenerative disease characterized by the destruction of neurons accompanied by memory loss and other cognitive deficits. Brain atrophy in Alzheimer’s disease often presents asymmetrically and is associated with loss of the normal brain asymmetries of development and ageing.110 Alzheimer’s disease brains also show asymmetric reductions in glucose metabolism, which correspond to neuropsychological symptom types.110 Studies show that grey matter atrophy seems to occur earlier and progress faster in the left hemisphere.30,31 Moreover, the asymmetric pathology may also be found in the neurite connectivity of the brain: one recent study found that core neuronal connections of the left hemisphere were depleted in Alzheimer’s disease compared to healthy controls and that with disease progression these neural network asymmetries became more exaggerated.111 Furthermore, studies investigating white matter networks found rightward topological asymmetry in later stages of the disease, with significant differences within only the left hemisphere.112 Interestingly, the apparent vulnerability of the left hemisphere in Alzheimer’s disease may in part be an accelerated form of normal ageing; cortical thinning is known to occur asymmetrically with age, resulting in a gradual loss of normal asymmetry, and this occurs more rapidly in Alzheimer’s disease.48 The prominent neuropathological features identified in Alzheimer’s disease are intracellular neurofibrillary tangles of phosphorylated tau protein and extracellular amyloid-beta (Aβ) plaques associated with neuronal dysfunction and death; these may also contribute to the phenotypic asymmetries seen in Alzheimer’s disease. PET scans as well as post-mortem studies of hemispheric distributions of these proteins in Alzheimer’s disease reveal asymmetric patterns of Aβ pathology in some cases.113–115 These asymmetric differences in neurodegeneration suggest a potential selective vulnerability in the left hemisphere to Alzheimer’s disease pathogenesis.
This observed asymmetry is also correlated with the clinical symptoms associated with Alzheimer’s disease pathology. Decreased efficiency in left hemisphere networks of Alzheimer’s disease patients is associated with poorer verbal memory.112 Left side asymmetry was also associated with poor verbal memory in mild cognitive impairment, the putative predecessor to Alzheimer’s disease.116 Paranoid delusions were shown specifically in Alzheimer’s disease patients with asymmetric temporal horns.117 Having more Aβ plaques in the left hemisphere is correlated with more severe language impairment, while rightward asymmetry in pathology is correlated with more severe visuospatial related symptoms.115 These correlations reinforce our understanding of the functional lateralization of the human brain and further implicate the left hemisphere in Alzheimer’s disease (Table 1). As in Parkinson’s disease, the origins of these asymmetries in Alzheimer’s disease remain a mystery.
Amyotrophic lateral sclerosis
ALS is a fatal, progressive neurodegenerative disease characterized by loss of both upper and lower motor neurons, resulting in motor dysfunction and ultimately, paralysis. ALS frequently presents with unilateral symptom onset, typically starting with limb weakness originating on one side of the body. Throughout disease progression, this pattern of degenerative spread remains observable as the area of initial onset remains the most affected.28 ALS patients also exhibit asymmetric grey matter atrophy.118 ALS pathology includes the aggregation of several different ubiquinated proteins in motor neurons, with TDP-43 found in the majority of patients.119 Although little research has been done on the asymmetric distribution of protein aggregation in ALS, in other neurodegenerative conditions, frontotemporal dementia and primary progressive aphasia, TDP-43 has been found to have asymmetric distribution patterns between brain hemispheres with greater densities in the language-dominant hemisphere (Table 1).120–122
Multiple sclerosis
MS is a demyelinating disease of the CNS that is progressive and potentially disabling. Owing to the variation in axonal damage, patients with MS vary in symptoms, including sensory disturbances, limb weakness, clumsiness and bowel dysfunction. Although the pathological mechanism of MS is still not known, it is largely believed to be an autoimmune-mediated mechanism where self-reactive immune cells destroy the myelin sheath, oligodendrocytes and axons.123 While not as distinctive as in Parkinson’s disease, one early clinical symptom of MS is unilateral limb weakness, and with disease progression, the symptom asymmetries in the limb remain.29,124 Studies investigating the limb asymmetries of MS have found performance differences between the legs of MS patients including workload and aerobic performance asymmetries.125 Furthermore, metabolic asymmetries of glucose uptake between limbs have also been shown.126 In each of these cases, the primary symptomatic limb showed decreased workload, aerobic performance and glucose uptake. In addition, early symptoms of relapsing-remitting MS often include inflammation of the optic nerve in only one eye.123 Taken together, these clinical differences between the right and left sides of the body suggest an underlying asymmetry in the CNS that contributes to this disease.
The characteristic lesions in MS typically have a mildly asymmetric distribution early in disease, though they eventually can be found on both sides.127 Neuroimaging studies investigating neurodegeneration in MS have found asymmetric grey matter loss in left brain regions including the left frontotemporal cortex, precuneus and cingulate gyrus,128 further supporting a possible left-brain vulnerability as in Alzheimer’s disease. Measures of corticospinal excitability in MS showed that the hemisphere contralateral to the weaker hand showed greater excitability, potentially indicating increased neuro-inflammation on one side; further, a shift in greater excitability to the other side predicted disease progression towards more symptom severity (Table 1).129
Given the heterogeneity and unpredictable nature of this disease, multiple pathways could contribute to disease pathogenesis, some of which could be influenced by inherent asymmetries. Focussing future research on clinical markers which are asymmetric at onset could help pinpoint triggers or underlying vulnerabilities that contribute to the early stages of MS. Perhaps with further investigation, the presence or degree of asymmetry could also be a useful biomarker to distinguish subtypes of MS.
Possible mechanisms for the impact of lateralization on neurodegenerative disease
Connections between handedness and neurodegenerative diseases
A growing body of evidence suggests a genetic link between brain asymmetry, handedness, and neurological disorders. Looking for an overlap in the genetic coding for lateralization and those associated with neurological disease may give insight into the pathological processes underlying these diseases. However, brain lateralization and handedness are complex phenotypes, making their origins difficult to unravel, as previously discussed.6,8 Despite the complexity, some intriguing patterns are beginning to emerge.
Studies suggest an association between handedness and neurodegenerative and neuropsychiatric diseases. Left-handed individuals experience a higher occurrence of various neuropsychological conditions, including depression, anxiety, bi-polar disorder, alcohol addiction and schizophrenia.6 In addition, left-handers show a greater response to drugs affecting the CNS.130 This phenomenon also extends to foot preference, with a higher prevalence of mixed and left footedness among individuals with neurodevelopmental and psychiatric disorders.55 Some have suggested handedness as a factor influencing the unilateral onset observed in Parkinson’s disease, and in several studies, the majority of right-handed patients have right-handed onset of symptoms.107,131,132 Indeed, studies have found that motor symptoms are more likely to be more severe in the dominant hand.107,131 However, the association of handedness is weak as shown by the occurrence of patients with non-dominant hand motor symptom onset, suggesting that other factors may also be at play.132,133 Studies have also found concordances between the dominant limb and side of symptom onset in ALS. One such study found 64% of a cohort of ALS patients had dominant side onset,134 while another found 70% of patients had dominant side onset.135 It was also shown that the side of limb onset was predictive of the asymmetry of grey matter losses observed in ALS.118 Furthermore, in another study, right-handed patients with ALS showed a disproportionate loss of grey matter in the left motor cortical hand area, while only those with a right-side (dominant side) symptom onset showed asymmetric atrophy in sensorimotor and language-related regions in the left hemisphere.136 While handedness is not well-studied in MS, one study did find a slightly increased risk for MS among left-handed women137 but this was not replicated in another, larger cohort138; no studies have explored correlations between side of symptom onset and handedness in MS. These patterns of handedness associated with neurodegenerative disease suggest that some common mechanism may be conferring vulnerability of one side of the brain and body to disease.
Recent GWAS studies have revealed that some of the same pathways that likely help establish asymmetry in the healthy human are those disrupted in neurodegenerative diseases. Many of these studies have focussed on neurodevelopmental disorders, revealing an association of significant loci that contribute to handedness and/or structural lateralization with those involved in schizophrenia, dyslexia, and autism spectrum disorder, but some of the loci identified are also associated with Parkinson’s disease and Alzheimer’s disease risk.66,75,79,81 These implicated genes include MAP2 and MAPT, which are microtubule-associated genes essential for neuronal development; the MAPT locus, which encodes for the protein tau, is a well-known region for both Alzheimer’s disease and Parkinson’s disease risk.139 In addition, microtubules contribute to axonal transport,140 the dysfunction of which may play a role in Parkinson’s disease, Alzheimer’s disease, ALS and MS.141,142 Another genetic study characterizing single-nucleotide polymorphisms (SNPs) found an association between previously identified Alzheimer’s disease risk SNPs and structural asymmetry of the brain. Two significant risk SNPs, at the TNKS and DLG2 loci, have been associated with subcortical volume differences in the putamen and amygdala, areas involved in learning and emotion, respectively.143 It should be noted that the two significantly associated SNPs are simply ‘associated’ with structural brain asymmetry and Alzheimer’s disease risk. They may not be causal, since other SNPs in the region in linkage disequilibrium may be surrogates of causal inference. Furthermore, the indicated genes at the loci may not exclusively (or at all) be involved in Alzheimer’s disease nor brain asymmetry risk. The results do, however, point to the loci being significantly involved in one way or another, which are interesting, informative, and may lead to hypotheses that can be tested.
As noted previously, the genetic pathways leading to structural asymmetries associated with language lateralization should not be automatically equated with those that contribute to handedness; indeed, a GWAS study identifying loci associated with planum temporale asymmetry found no overlap with risk SNPs for schizophrenia, autism spectrum disorder or ADHD.144 Nevertheless, taken together, significant evidence suggests the existence of an underlying process in the neurodevelopment of motor dominance which provides—at least in part—insights into the origins of asymmetry in disease. Mechanisms for how these common pathways might lead to asymmetric disease onset will be further explored in later in the review.
In addition to common developmental pathways between handedness and neurodegenerative disease, handedness may impact vulnerability to disease through the accumulated impact of biased use over a lifetime. This limb dominance theory is a prevailing explanation for the unilateral symptom onset observed in ALS. It has been suggested that strenuous activity may increase the risk of ALS potentially through increased oxidative damage.145,146 In the upper limb, strenuous activity is often biased to one side of the body due to handedness. This hypothesis suggests that the dominant limb and thus the contralateral brain hemisphere are at an increased vulnerability of disease onset due to increased use over a lifetime. The dominant limb hypothesis may also suggest subtle differences in innervation or excitability of the upper limb which may also contribute to the marked asymmetry of ALS.134 The asymmetric pattern is less common in the lower limb; it has been proposed that although there is also inherent ‘footedness’, throughout life the lower limbs receive relatively equal use134; this adds credence to the theory that increased use confers vulnerability. However, despite the strong evidence of the involvement of neural connectivity of the dominant upper limb, dominance of symptoms in the upper limb is not a pathological feature of all individuals with ALS. This theory has not received much attention in other neurodegenerative diseases, but oxidative stress is implicated in Parkinson’s disease progression,147 and thus increased use of one limb over time may exacerbate mitochondrial dysfunction and oxidative stress and thus aggravate disease progression faster in one hemisphere.148,149
It is also worth noting that research investigating connections between handedness, structural asymmetry, and neurological disease may be confounded by inconsistent measures of handedness, inaccurate reporting of side of symptom onset (influenced by poor patient recall), and/or cultural biases that influence hand use. For these reasons, some have suggested other measures of brain lateralization such as foot preference to be more accurate reflections of neurological lateralization, which should be considered in future clinical reporting and studies investigating this intriguing connection.54,55
Epigenetics sets the stage for asymmetric neurodegeneration
Like the genetic loci associated with handedness, epigenetic differences between hemispheres that are involved in the development of brain lateralization may also confer vulnerability to neurodegenerative diseases. As discussed previously (See ‘The Development of the Asymmetric Brain’), CpH methylation in prefrontal cortex neurons varies across hemispheres, with increased methylation in the left hemisphere of healthy individuals.93 Interestingly, in this study, the loci showing differential methylation were associated with neurological diseases including neurodegenerative diseases. Our findings suggest that epigenetics could play a large and significant role in hemispheric vulnerabilities related to disease (Fig. 1).
Figure 1.
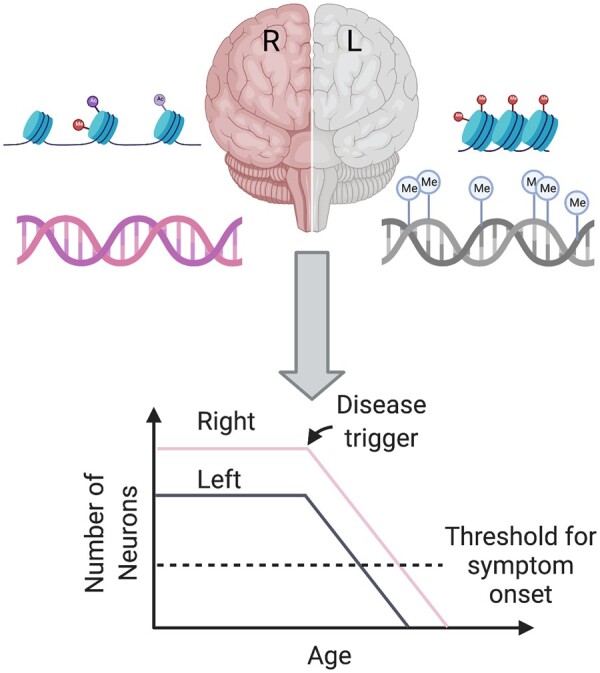
Epigenetic asymmetry sets the stage for asymmetric disease. Epigenetic modifications such as histone markers and methylation (denoted by ‘Me’) affect chromatin accessibility and gene expression. These modifications may be asymmetric from birth or with ageing and result in different cytoarchitecture and immune profiles between hemispheres. For example, decreased neurogenesis in one hemisphere may result in fewer neurons in the left hemisphere, which could set the stage for asymmetric neurodegeneration and unilateral symptom onset.
Epigenetics has been a topic of research to better understand the asymmetry of Parkinson’s disease. Studies investigating genetic variance in monozygotic twins suggest that the side of motor symptom onset is sporadic and found no genetic explanation for this asymmetric phenomenon.27 Further evidence for the lack of a wholly genetic explanation of asymmetry can be drawn from work in familial Parkinson’s disease cases (which are caused by a known genetic mutation) where the side of onset was found to be random.27 For course, as stated previously, the primary DNA sequence is the same in the two brain hemispheres but not the chromatin as exemplified by epigenetics. The Labrie lab has found that hemispheric asymmetry in the epigenome of neurons was associated with the lateralization of Parkinson’s disease symptoms in two independent cohorts.93 These epigenetic abnormalities were most abundant in the hemisphere contralateral to symptom onset. Specifically, the left hemisphere of Parkinson’s-affected individuals has greater epigenetic dysregulation, which affected genes involved in immune activation, inflammation, neuronal development, glucose homeostasis, protein localization and synaptic activity. Transcriptomic and proteomic analysis aligned with these results, showing asymmetries in similar pathways.93 Asymmetry in Parkinson’s disease was also shown in regulatory elements of Parkinson’s disease Risk genes. These epigenetic abnormalities provide support to the hypothesis that hemispheric differences in Parkinson’s disease individuals create unilateral vulnerabilities.93 Our epigenetic analysis also addressed disease progression and suggested that methylation asymmetries were more pronounced in Parkinson’s disease patients, and Parkinson’s disease patients with highly lateralized symptoms have slower disease progression than those with symmetrical symptoms.93 Despite these epigenetic associations, an important question remains: are these findings causes or consequence of disease? Further epigenetic analysis looking at broader chromatin structure with techniques such as ATAC-seq and investigating epigenetic asymmetries in younger individuals may provide more understanding of asymmetry in Parkinson’s disease and other neurodegenerative diseases. Currently, no epigenetic studies have characterized the epigenome in Alzheimer’s disease, ALS, and MS in the context of asymmetry, but such studies could similarly reveal key pathways that may be involved in disease risk. Epigenetic differences between hemispheres that exist early in develoment or change over time may contribute to asymmetries in disease development and onset later in life (Fig. 1).
It is further possible that at least some differential gene expression between brain hemispheres, potentially driven by these epigenetic differences, could contribute to differences in disease development. As we have seen, differences in gene expression exist between hemispheres in both adult humans and during foetal development.80,95–97 It is well-known that dynamic regulation of gene expression in general occurs during cellular differentiation, as was recently exemplified in time-series RNA sequencing data, over 16 time points during the differentiation of induced pluripotent stem cells to cardiomyocytes.150 Therefore, differences in gene expression may ‘set-up’ regions of the brain as a first hit for future vulnerabilities to other subsequent insults.151 In the following sections, we will suggest how this regulation may contribute to asymmetric vulnerabilities through cytoarchitecture, protein distribution and connectivity.
Asymmetry in cytoarchitecture
Beyond gross anatomical asymmetries, the distributions of cells may differ between hemispheres. Epigenetic differences between hemispheres may create a blueprint for an imbalance of neurons or glial cells, which could have serious implications for the pathogenesis and progression of neurodegenerative disease later in life. Unfortunately, most studies that have undertaken a cell count in the healthy human brain have only counted one hemisphere or averaged the two sides, leaving a conspicuous gap in our knowledge of innate CNS asymmetry. Some have proposed an inherent deficit of dopaminergic neurons in one side of the SN as an explanation for the unilateral motor symptom onset in Parkinson’s disease; however, the few studies that have counted dopaminergic neurons in both hemispheres of healthy individuals used inconsistent methodology and produced differing results.27 One study using neuromelanin imaging found a significant loss in the left SN even in healthy controls, indicating a potential left side vulnerability in Parkinson’s disease.152 A review investigating dopaminergic neuron counts between healthy individuals found a high individual variation and suggested a degree of risk associated with lower dopaminergic neuronal counts153; however, hemispheric dopaminergic neuronal counts were not investigated. As differences in neuronal abundance between hemispheres has the potential to largely influence asymmetric pathogenesis of disease, future research quantifying neurons between hemispheres should be done.
Among the genes differentially expressed in the developing human brain is LMO4, which is more highly expressed in the right perisylvian region between 12 and 17 weeks development96; this gene is likely a transcriptional regulator crucial for neurodevelopment, and unilateral knockout in mice has been shown to cause asymmetric neurogenesis and modulate lateralized behaviour.154 Though more work is needed to translate this work to humans, this illustrates in principle that the genetic and epigenetic asymmetries in humans could leave one side of the brain with fewer neurons and thus more likely to reach a threshold of deficits that manifest in unilateral symptoms.
The resident immune cells of the brain (microglia) and neuronal support systems are also established in neurodevelopment, but little is known about whether glial cells exhibit asymmetry in distribution or function. Significant regional heterogeneity has been found in the distribution of microglia, oligodendrocytes, and astrocytes in both mice and humans,155 but like neurons, in studies quantifying this distribution, effort has not been made to consider differences between hemispheres. Microglia appear to populate the brain during development from several origins and in multiple waves which may take different routes into the developing brain; their distribution seems to be influenced by local differences in neuronal connections, supporting glia, and cytokines.155 It stands to reason that the same factors governing the process of lateralization for various structures and connectivity in the brain could also influence an asymmetric distribution of microglia. This is of particular importance to study given that microglia activation is implicated in neurodegeneration in Parkinson’s disease, Alzheimer’s disease, ALS and MS,156 and thus a higher density of activated microglia in one hemisphere could contribute to asymmetric initiation or progression of pathology.
One key player in the distribution of cells may be the choroid plexus (CP). The CP is a group of specialized cells located in the cerebral ventricles and is one of the first structures to show lateralization in human development.97 In adults, the CP is responsible for production of CSF and serves as the entry point for immune cells into the CSF (Fig. 2).157 Studies have shown that the CP plays a crucial role during neurodevelopment and in immunosurveillance; microglia appear to first enter the CSF through the CP.155,158 Early in development, the left CP expresses more genes involved in cell adhesion and immune response, while the right expresses more cilia-related genes, which serve to circulate CSF in the ventricles.80 The flow of CSF directs the migration of neuroblasts during development.159 Thus these early differences could lead to inherent asymmetries in CSF production and circulation and the blood–CSF barrier, which may impact the distribution of neurons and resident immune cells, though these potential consequences have yet to be investigated. In addition, the CP is involved in the maintenance of neurogenesis in adulthood in the ventricular–subventricular zone and the sub granular zone of the hippocampus157; differences in the CP in the left and right ventricle could thus theoretically modify the number of neurons in each hemisphere.
Figure 2.
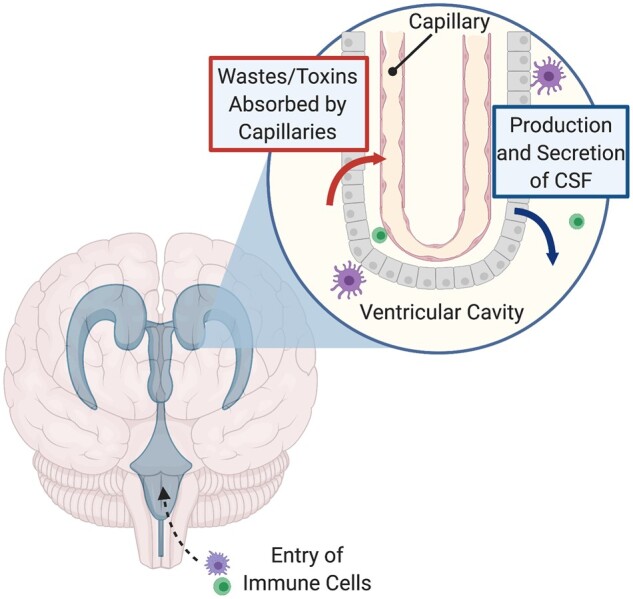
Anatomy of the choroid plexus (CP). The CP lies within the cerebral ventricles, including the right and left lateral ventricles. A segment of the CP is shown above, showing the specialized epithelial cells surrounding fenestrated capillaries where wastes and toxins are absorbed from ventricular cavities. The CP secretes fresh CSF into the cerebral ventricles to continue to protect the CNS. The CP also houses specialized immune cells and serves as an entry point for immune cells into the CNS. Figure adapted from Lun et al.157
Although the pathogenesis of MS is not well understood, the autoimmune nature of this disease suggests that asymmetries in the CP may be another mechanism for asymmetric pathology. MS Lesion patterns have been associated with differences in T-Helper cell distributions,160 which could vary between hemispheres. Immune cell entry to the CNS is strictly regulated by the blood–brain barrier (BBB) and the blood–CSF barrier (namely the CP), and thus dysfunctions in these systems could contribute to MS.158 If this dysfunction occurs selectively in one hemisphere, this could help explain the asymmetric manifestations of disease; however, limited research in this area exists. In rats, BBB breakdown begins in the hemisphere contralateral to paw preference and occurs more rapidly in the left hemisphere of right paw preference rats.161 Correlations of MS lesion patterns with handedness may thus reveal interesting patterns. Similarly, differences in the right and left CP (Fig. 2) may contribute to an asymmetric passage of self-reactive immune cells and T-Helper cells associated with MS to the CNS, resulting in asymmetric lesions and grey matter loss.
Thus, asymmetric cytoarchitecture established by the CP or other mechanisms may create a unilateral vulnerability to neurodegeneration due to a lower number of neurons or a greater number of microglia and other immune cells.
Neurochemical asymmetry
The brain may also have innate neurochemical differences between the hemispheres that contribute to unilateral vulnerability. In one study that quantified neurotransmitters in the left and right hemispheres of 9 brain regions, left–right differences were found only in gamma aminobutyric acid (GABA) in the nigra, but there was large variability between samples.162 Further analysis revealed compelling evidence for neurochemical laterality with individual brains showing different degrees of asymmetry overall: left–right asymmetries in glutamic acid decarboxylase and GABA were positively correlated across structures.163 Brains that were asymmetric in one region were more likely to be asymmetric in another as well, and within a structure, asymmetries of different neurotransmitters were more likely to be in the same direction. Choline acetyltransferase (CHAT) and dopamine (DA) were also both greater in the left globus pallidus.163 In this dataset, the largest differences appear to occur in the nigrostriatal regions, and subsequent work has confirmed that dopaminergic pathways show clear hemispheric differences.
Hemispheric differences in the nigrostriatal DA pathway have clear relevance for Parkinson’s disease given that motor symptom onset is ultimately caused by a loss of dopaminergic neurons in the SN. In Parkinson’s disease, PET scans using fluorine-18-labelled fluorodopa consistently show reduced DA uptake in the posterior putamen contralateral to symptom onset; in fact, abnormal asymmetric uptake of DA has been found in close relatives of Parkinson’s disease patients who had not yet developed motor symptoms.27 In healthy individuals, right–left differences have been found in measures of DA, the concentration of D1 and D2 receptors, and the binding potential of DA receptors and transporters in the striatum, neocortex, and nucleus accumbens.164,165 Furthermore, the natural loss of striatal DA transporters with age appears to occur evenly on both sides, preserving this asymmetry in older individuals.166 These differences in the dopaminergic pathways correlate with various functional asymmetries including paw and rotation preference in mice.164 Likewise, asymmetry in the nigrostriatal dopaminergic system seems to be a key factor in the lateralization of motor behaviour in humans; in one study, the degree of right-hand preference was correlated to greater fluorodopa uptake in the left putamen.167 This suggests that naturally occurring neurochemical asymmetries in the nigrostriatal pathway may contribute to handedness and may also set up one hemisphere for greater risk of DA deficits in neurological disease. These inherent DA asymmetries have been well studied in schizophrenia, which seems to involve a pathological abnormality of DA21,168–172; the findings of the role of asymmetry in this disease may also be relevant to Parkinson’s disease.
Asymmetrical influences on pathological protein spread
Several neurodegenerative diseases including Parkinson’s disease, Alzheimer’s disease and ALS involve pathological aggregates of protein in the brain which spread as the disease progresses. The spread of these proteins may be influenced by the amount of endogenous protein template or by the connectivity patterns found in the brain; thus, inherent asymmetries in both these factors may influence neurodegeneration and inform our understanding of disease progression.
As discussed previously, Parkinson’s disease is characterized by misfolded α-syn aggregates into intracellular inclusions known as Lewy bodies as well as insoluble fibrils found mostly near axons (Lewy neurites). These pathologic aggregates follow a stereotypical spread originating in the brainstem in the dorsal motor nucleus of the vagus nerve and the olfactory bulb and eventually spreading to the prefrontal cortex in late stages of the disease,103 and some patients exhibit more α-syn pathology in the hemisphere corresponding to motor symptom dominance.104 It is worth noting that α-syn aggregates have been found in healthy elderly individuals, with autopsy revealing slight asymmetries between Lewy pathologies in the left and right brain stem.173 The histological hallmarks of Alzheimer’s disease include tau protein ‘tangles’ and Aβ plaques. Like Parkinson’s disease, these proteins follow a stereotypical spread pattern through the brain as the disease progresses, starting from the transentorhinal cortex for tau and basal portions of the isocortex for Aβ.174 PET scans as well as post-mortem studies of hemispheric distributions of these proteins in Alzheimer’s disease reveals asymmetric patterns of Aβ pathology in some cases.113–115 In ALS, aggregated ubiquinated TDP-43 protein is found in the majority of patients, sometimes with other ubiquinated proteins.119 TDP-43 has been shown to have an asymmetric distribution pattern with greater density in the language dominant hemisphere in other neurodegenerative conditions including frontotemporal dementia and primary progressive aphasia.120–122
Since α-syn, tau, Aβ and TDP-43 misfolding follow a pattern of prion-like spread,175–177 the amount of endogenous protein template could predispose one hemisphere to pathology. An in vitro model has shown that susceptibility of neuron types to α-syn spread is linked to their relative expression of SNCA, the gene that codes for α-syn.178 Similarly, in a rat model, researchers traced the spread of human α-syn after injection and found that selective vulnerability of brain regions to the spread of synucleinopathy and neuronal death was closely correlated with regional expression of α-syn.179 In addition, this study showed that the spread was correlated with neuronal connectivity.179
Global asymmetric connectivity patterns may also influence asymmetric spread of protein. As discussed previously, healthy humans exhibit significant asymmetrical patterns in neurite dispersal patterns and density.52 Modelling has shown that a pattern of transneuronal spread best predicts the distribution of tau and Aβ in the brains of Alzheimer’s disease patients,180 and α-syn and TDP-43 are known to be transmitted across axon terminals177,181. In addition, connectivity networks can predict vulnerability of certain regions to neurodegeneration.182 Thus, we hypothesize that the patterns of microstructural asymmetry established during development may create unilateral vulnerabilities to pathological protein aggregation.
Detailed comparison of the expression and pathology of these proteins between hemispheres in human post-mortem brain has yet to be undertaken, but such studies could help explain regional asymmetric vulnerability to these diseases. Furthermore, the existence of asymmetrical pathology in patients with these diseases has critical implications for the pathological staging of human post-mortem samples used in research. In the case of Parkinson’s disease and Alzheimer’s disease, Braak staging is often used as selection criteria and as a covariate in statistical tests, yet the tissue used in the study may be from the opposite hemisphere used for the histological analysis and thus may not accurately reflect the reported stage of disease.
The asymmetric arrival of a neurodegenerative trigger
While the previous hypotheses have suggested ways that inherent asymmetries in the CNS may predispose one hemisphere to disease pathogenesis and progression, peripheral factors may also cause or contribute to asymmetric neurodegeneration. Numerous studies have suggested a role for the microbiome and infectious triggers in neurodegenerative disease; while this is largely outside the scope of this review, two key entrance points from the periphery suggest a mechanism by which a neurodegenerative disease trigger could reach only one side of the brain, potentially leading to the asymmetric symptoms observed.
The first key pathway is the asymmetric vagal nerve. In Parkinson’s disease, gastrointestinal symptoms precede motor symptom onset by decades and increasing evidence suggests that Parkinson’s disease may sometimes begin in the periphery.183 Mounting evidence suggests that the vagus nerve could form a highway for pathology to travel to the brain in Parkinson’s disease. α-Syn can spread through neurons in a prion-like fashion and has been shown to travel to the brain through the vagal nerve in mice.184,185 Furthermore, epidemiological studies have shown that truncal vagotomy or appendectomy are associated with a lower risk of Parkinson’s disease.186,187 Interestingly, the vagus nerve presents an asymmetrical path between the gut and the brain. Although discussed as singular, the vagus nerve consists of both left and right branches which emerge from the medulla and follow differing paths throughout the body: most notably, the right branches innervate the intestines while the left branches form the hepatic plexus on the liver. If Parkinson’s disease pathology is seeded from the gut, the divergent pathways of the vagus nerves could result an asymmetric arrival on either side of the brainstem depending on the initiation site of the pathology (Fig. 3). Whether the spread of α-syn would remain unilateral within the brain depends on the patterns of connectivity to other regions of the brain (as discussed in the previous section). In one study of α-syn spread in mice, recombinant adeno-associated viral vectors expressing human α-syn were injected unilaterally into the left vagus nerve to induce spread through retrograde viral transport. After 18 weeks, α-syn pathology was found on both sides of the brain, but the right hemisphere only had about 35% as much pathology as the left.185 This suggests that α-syn entering the brain through a vagal route would lead to more prominent degeneration in one hemisphere, potentially causing the unilateral motor symptom onset that is characteristic of Parkinson’s disease. Although this mechanism has been most well-studied in the context of Parkinson’s disease and α-syn, the vagal nerve could also theoretically serve as a pathway for the asymmetric arrival of other neurodegenerative triggers such as viruses.188
Figure 3.
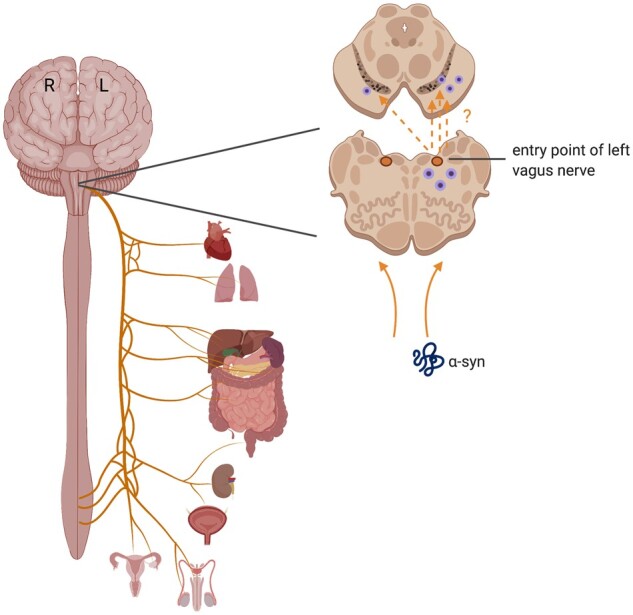
Asymmetric vagus nerve pathways. Though often discussed in the singular, the vagus nerve consists of right and left branches, which each innervate different organs. Retrograde spread of misfolded α-syn from the periphery may thus show an asymmetric introduction and spread within the brain depending on its origin.
Secondly, the BBB or blood–CSF barrier could also serve as an asymmetric entry point for a pathogenic trigger. Asymmetric features of neurological diseases fit well with recent theories suggesting that neurodegeneration can be triggered by a direct infection by a virus or other pathogen. Viral or bacterial infection has been hypothesized to play a role in Alzheimer’s disease,189–191 Parkinson’s disease,149,192 MS123 and ALS.193,194 A pathogen travelling to the brain via peripheral nerves through retrograde transport or through blood circulation must first infect a specific region of the brain. Differences in cerebral blood flow between hemispheres or a weakening of the BBB or blood–CSF barrier on one side could leave one hemisphere more vulnerable to infection. Cerebral blood flow is indeed asymmetric, with the left hemisphere overall receiving lower flow.195 Some show that differences between hemispheres in cerebral blood flow become more pronounced with age, and there is a deficit of CBF in the left frontal and temporal lobes in older individuals.195–198 In rats, BBB breakdown begins in the hemisphere contralateral to paw preference and occurs more rapidly in the left hemisphere of right paw preference rats.161 Whether this asymmetry in BBB breakdown exists in human is unknown. Asymmetries in the blood–CSF barrier are also possible given the early lateralization seen in the CP (see ‘Asymmetry in Cytoarchitecture’), and since some viruses implicated in neurodegeneration use a ‘trojan horse’ method to enter the brain within immune cells,188,194 the CP may be an asymmetric entry point.
Interestingly, in two recent cases of parkinsonism after SARS-COV-2 infection, patients demonstrated characteristic unilateral motor symptoms, and PET scans demonstrated corresponding asymmetric deficits in DA uptake199,200; though a causal link between the virus and these symptoms cannot be definitively established, this adds evidence to the possibility of direct viral infection in the CNS leading to asymmetric neurodegeneration. Furthermore, systemic infection earlier in life could induce inflammation which is more severe in specific regions of the brain based on microglia distribution (see ‘Asymmetry in Cytoarchitecture’) or cerebral blood flow differences, which could ‘prime’ one hemisphere for subsequent risk.
Implications for future research
Given the asymmetry in the healthy CNS and the commonality of asymmetry across neurodegenerative diseases, viewing future research through this lens may yield insightful results. For one, future genetic, transcriptomic and epigenetic studies would do well to consider hemisphere of origin in their samples and analyses; grouping tissue from different hemispheres together may be masking or diluting significant results that may only be present in the more vulnerable hemisphere. Future cell count and imaging studies should also consider quantifying and comparing both hemispheres to build our understanding of difference between hemispheres in both healthy and diseased individuals, which may point to new avenues of prevention and treatment. Most recently, the revelation of the brain glymphatic system suggests another aspect of the brain which could have asymmetric vulnerabilities201; given the role of this system in waste clearance and immune cell trafficking, inherent asymmetries in lymphatic flow in the brain or a unilateral dysfunction in this system could contribute to asymmetric disease pathogenesis.202–204 Indeed, a recent study found impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease; however, although drainage was quantified by MRI for each hemisphere separately and appears asymmetric in representative images, the bilateral data were combined for each individual in the study’s reporting and analysis, erasing potential insights into lateralization of this impairment.205 The blood–CSF barrier similarly holds intriguing possibilities for study, especially given the asymmetries shown early in development in the CP (Fig. 2).80,97 Finally, in vivo work should also take asymmetry into account, although further work is needed to determine the extent to which rodent models accurately mirror human CNS lateralization. In particular, models investigating the role of the gastrointestinal tract in neurological disease should consider if pathologic spread to the brain is asymmetric; in addition to quantifying pathology on both sides of the hemisphere, determining paw-preference or other spatial preferences before disease onset may also reveal interesting correlations. In Parkinson’s disease studies, behavioural tests such as the cylinder test or catwalk can also be added to the testing regiment to detect possible motor asymmetries, which may add further insight into model accuracy and hemispheric vulnerability.206 Since asymmetry has been often neglected in studies of neurodegenerative disease, we believe this perspective may illuminate exciting new insights.
Conclusion
In summary, current knowledge of asymmetries in neurodegenerative diseases provides evidence that phenotypic asymmetries present based on inherent vulnerabilities in one hemisphere. The human CNS exhibits structural and functional asymmetry which appears early in development. Epigenetic regulation and gene expression differs between hemispheres which could drive asymmetries in cellular distribution, connectivity, neurotransmitters and protein expression. These inherent differences may set up one hemisphere for disease pathogenesis. In addition, asymmetric paths from the periphery may selectively introduce a pathological trigger to one side of the brain, precipitating or exacerbating the unilateral onset of disease. Though neurodegenerative diseases are heterogenous, even within a disease type, clinical asymmetry can be found in Parkinson’s disease, Alzheimer’s disease, ALS and MS. Hemispheric differences have been often disregarded in studies of these diseases, yet delving into this enigma of asymmetry may provide insight into their aetiology and unlock new therapeutic potential. At the least, considering hemisphere of origin as an important variable in future studies using human post-mortem tissue and recognizing hemispheric differences in pathological staging may strengthen research in neurodegenerative disease.
Acknowledgements
We dedicate this review in memory of our research advisor, mentor, colleague and friend, Dr Viviane Labrie. This review was driven by her vision and creativity; may it inspire others to carry on this work. Figures were created using BioRender. We also thank the extensive positive critique and suggestions received from the reviewers of this manuscript; it significantly improved the paper.
Funding
This work was supported by a grant from the National Institutes of Health (NIH R01NS113894).
Competing interests
The authors report no competing interests.
Data availability
Data sharing is not applicable to this article as no new data were created or analysed.
Glossary
- ALS
amyotrophic lateral sclerosis
- Aβ
amyloid-beta
- α-syn
alpha-synuclein
- CP
choroid plexus
- BBB
blood–brain barrier
- DA
dopamine
- ENIGMA
enhancing neuro-imaging genetics through meta-analysis
- GWAS
genome-wide association studies
- MS
multiple sclerosis
- SN
substantia nigra
- SNP
single-nucleotide polymorphism
References
- 1.Broca PP.Perte de la parole, ramollissement chronique et destruction partielle du lobe antérieur gauche du cerveau (Loss of speech, chronic softening and partial destruction of the anterior left lobe of the brain). Bull Soc Anthropol. 1861;2:235–238. [Google Scholar]
- 2.Wernike C.Der aphasische Symptomencomplex; eine psychologische Studie auf anatomischer Basis. Cohn & Weigert: Breslau; 1874. [Google Scholar]
- 3.Sperry RW.Cerebral organization and behavior: The split brain behaves in many respects like two separate brains, providing new research possibilities. Science. 1961;133(3466):1749–1757. [DOI] [PubMed] [Google Scholar]
- 4.Gazzaniga MS.Principles of human brain organization derived from split-brain studies. Neuron. 1995;14(2):217–228. [DOI] [PubMed] [Google Scholar]
- 5.Kimura D, Archibald Y.. Motor functions of the left hemisphere. Brain. 1974;97(2):337–350. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz J, Metz GAS, Güntürkün O, Ocklenburg S.. Beyond the genome-Towards an epigenetic understanding of handedness ontogenesis. Prog Neurobiol. 2017;159:69–89. [DOI] [PubMed] [Google Scholar]
- 7.Duboc V, Dufourcq P, Blader P, Roussigné M.. Asymmetry of the brain: Development and implications. Annu Rev Genet. 2015;49:647–672. [DOI] [PubMed] [Google Scholar]
- 8.Güntürkün O, Ocklenburg S.. Ontogenesis of lateralization. Neuron. 2017;94(2):249–263. [DOI] [PubMed] [Google Scholar]
- 9.Gotts SJ, Jo HJ, Wallace GL, Saad ZS, Cox RW, Martin A.. Two distinct forms of functional lateralization in the human brain. Proc Natl Acad Sci U S A. 2013;110(36):E3435–E3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallortigara G, Rogers LJ.. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav Brain Sci. 2005;28(4):575–589.discussion 589–633. [DOI] [PubMed] [Google Scholar]
- 11.Rogers LJ, Zucca P, Vallortigara G.. Advantages of having a lateralized brain. Proc Biol Sci. 2004;271 (Suppl 6):S420–S422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corballis MC.The evolution of lateralized brain circuits. Front Psychol. 2017;8:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mundorf A, Ocklenburg S.. The clinical neuroscience of lateralization; Routledge: Abingdon, Oxon; 2021. [Google Scholar]
- 14.Kong XZ, Postema MC, Guadalupe T, et al. Mapping brain asymmetry in health and disease through the ENIGMA consortium. Hum Brain Mapp. 2020;doi: 10.1002/hbm.25033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postema MC, van Rooij D, Anagnostou E, et al. Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nat Commun. 2019;10(1):4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oertel-Knöchel V, Linden DE.. Cerebral asymmetry in schizophrenia. Neuroscientist. 2011;17(5):456–467. [DOI] [PubMed] [Google Scholar]
- 17.Crow TJ.Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20(8):339–343. [DOI] [PubMed] [Google Scholar]
- 18.Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G.. Abnormal asymmetry of brain connectivity in schizophrenia. Front Hum Neurosci. 2014;8:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruzelier JH.Functional neuropsychophysiological asymmetry in schizophrenia: A review and reorientation. Schizophr Bull. 1999;25(1):91–120. [DOI] [PubMed] [Google Scholar]
- 20.Vita A, De Peri L, Deste G, Sacchetti E.. Progressive loss of cortical gray matter in schizophrenia: A meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A.. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: Meta-analysis. Br J Psychiatry. 2001;178:344–351. [DOI] [PubMed] [Google Scholar]
- 22.Bartolomeo P, Thiebaut de Schotten M.. Let thy left brain know what thy right brain doeth: Inter-hemispheric compensation of functional deficits after brain damage. Neuropsychologia. 2016;93(Pt B):407–412. [DOI] [PubMed] [Google Scholar]
- 23.Xiaoli G, Wenqing W, Shanbao T.. Asymmetry of hemispheric interdependences in the early hours following unilateral stroke: An electrophysiological study in rats. In: 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE: Jeju, South Korea; 2017. 4363–4366. doi: 10.1109/embc.2017.8037822 [DOI] [PubMed] [Google Scholar]
- 24.Bartur G, Pratt H, Frenkel-Toledo S, Soroker N.. Neurophysiological effects of mirror visual feedback in stroke patients with unilateral hemispheric damage. Brain Res. 2018;1700:170–180. [DOI] [PubMed] [Google Scholar]
- 25.Keser Z, Meier EL, Stockbridge MD, Breining BL, Sebastian R, Hillis AE.. Thalamic nuclei and thalamocortical pathways after left hemispheric stroke and their association with picture naming. Brain Connect. 2021;doi: 10.1089/brain.2020.0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch G, Veniero D, Caltagirone C.. To the other side of the neglected brain: The hyperexcitability of the left intact hemisphere. Neuroscientist. 2013;19(2):208–217. [DOI] [PubMed] [Google Scholar]
- 27.Djaldetti R, Ziv I, Melamed E.. The mystery of motor asymmetry in Parkinson's disease. Lancet Neurol. 2006;5(9):796–802. [DOI] [PubMed] [Google Scholar]
- 28.Körner S, Kollewe K, Fahlbusch M, et al. Onset and spreading patterns of upper and lower motor neuron symptoms in amyotrophic lateral sclerosis. Muscle Nerve. 2011;43(5):636–642. [DOI] [PubMed] [Google Scholar]
- 29.Farrell JW, Motl RW, Learmonth YC, Pilutti LA.. Persons with multiple sclerosis exhibit strength asymmetries in both upper and lower extremities. Physiotherapy. 2020;111:83–91. [DOI] [PubMed] [Google Scholar]
- 30.Janke AL, de Zubicaray G, Rose SE, Griffin M, Chalk JB, Galloway GJ.. 4D deformation modeling of cortical disease progression in Alzheimer's dementia. Magn Reson Med. 2001;46(4):661–666. [DOI] [PubMed] [Google Scholar]
- 31.Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23(3):994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson PM, Mega MS, Woods RP, et al. Cortical change in Alzheimer's disease detected with a disease-specific population-based brain atlas. Cereb Cortex. 2001;11(1):1–16. [DOI] [PubMed] [Google Scholar]
- 33.2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020; 16(3): 391–460 [Google Scholar]
- 34.Ascherio A, Schwarzschild MA.. The epidemiology of Parkinson's disease: Risk factors and prevention. Lancet Neurol. 2016;15(12):1257–1272. [DOI] [PubMed] [Google Scholar]
- 35.Marras C, Beck JC, Bower JH, et al. ; Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arthur KC, Calvo A, Price TR, Geiger JT, Chiò A, Traynor BJ.. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat Commun. 2016;7:12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collaborators G.Global, regional, and national burden of multiple sclerosis 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toga AW, Thompson PM.. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4(1):37–48. [DOI] [PubMed] [Google Scholar]
- 39.Chi JG, Dooling EC, Gilles FH.. Gyral development of the human brain. Ann Neurol. 1977;1(1):86–93. [DOI] [PubMed] [Google Scholar]
- 40.Kasprian G, Langs G, Brugger PC, et al. The prenatal origin of hemispheric asymmetry: An in utero neuroimaging study. Cereb Cortex. 2011;21(5):1076–1083. [DOI] [PubMed] [Google Scholar]
- 41.Kong XZ, Mathias SR, Guadalupe T, et al. ; ENIGMA Laterality Working Group. Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. Proc Natl Acad Sci U S A. 2018;115(22):E5154–e5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guadalupe T, Mathias SR, vanErp TGM, et al. Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging Behav. 2017;11(5):1497–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Concha ML, Wilson SW.. Asymmetry in the epithalamus of vertebrates. J Anat. 2001;199(Pt 1-2):63–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianco IH, Wilson SW.. The habenular nuclei: A conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009;364(1519):1005–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R.. The parahippocampal gyrus in Alzheimer's disease. Clinical and preclinical neuroanatomical correlates. Ann N Y Acad Sci. 2000;911:254–274. [DOI] [PubMed] [Google Scholar]
- 46.Stranahan AM, Mattson MP.. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer's disease. Neural Plast. 2010;2010:108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazure CM, Swendsen J.. Sex differences in Alzheimer's disease and other dementias. Lancet Neurol. 2016;15(5):451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roe JM, Vidal-Piñeiro D, Sørensen Ø, et al. ; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. Asymmetric thinning of the cerebral cortex across the adult lifespan is accelerated in Alzheimer’s disease. Nat Commun. 2021;12(1):721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cullen TJ, Walker MA, Eastwood SL, Esiri MM, Harrison PJ, Crow TJ.. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br J Psychiatry. 2006;188:26–31. [DOI] [PubMed] [Google Scholar]
- 50.Smiley JF, Rosoklija G, Mancevski B, et al. Hemispheric comparisons of neuron density in the planum temporale of schizophrenia and nonpsychiatric brains. Psychiatry Res. 2011;192(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chance SA, Sawyer EK, Clover LM, Wicinski B, Hof PR, Crow TJ.. Hemispheric asymmetry in the fusiform gyrus distinguishes Homo sapiens from chimpanzees. Brain Struct Funct. 2013;218(6):1391–1405. [DOI] [PubMed] [Google Scholar]
- 52.Schmitz J, Fraenz C, Schlüter C, et al. Hemispheric asymmetries in cortical gray matter microstructure identified by neurite orientation dispersion and density imaging. Neuroimage. 2019;189:667–675. [DOI] [PubMed] [Google Scholar]
- 53.Kong XZ, Postema M, Schijven D, et al. Large-scale phenomic and genomic analysis of brain asymmetrical skew. Cereb Cortex. 2021;31(9):4151–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tran US, Stieger S, Voracek M.. Evidence for general right-, mixed-, and left-sidedness in self-reported handedness, footedness, eyedness, and earedness, and a primacy of footedness in a large-sample latent variable analysis. Neuropsychologia. 2014;62:220–232. [DOI] [PubMed] [Google Scholar]
- 55.Packheiser J, Schmitz J, Berretz G, et al. Four meta-analyses across 164 studies on atypical footedness prevalence and its relation to handedness. Sci Rep. 2020;10(1):14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barut C, Ozer CM, Sevinc O, Gumus M, Yunten Z.. Relationships between hand and foot preferences. Int J Neurosci. 2007;117(2):177–185. [DOI] [PubMed] [Google Scholar]
- 57.Güntürkün O.Human behaviour: Adult persistence of head-turning asymmetry. Nature. 2003;421(6924):711. [DOI] [PubMed] [Google Scholar]
- 58.Papadatou-Pastou M, Ntolka E, Schmitz J, et al. Human handedness: A meta-analysis. Psychol Bull. 2020;146(6):481–524. [DOI] [PubMed] [Google Scholar]
- 59.Papadatou-Pastou M, Martin M, Munafò MR, Jones GV.. Sex differences in left-handedness: A meta-analysis of 144 studies. Psychol Bull. 2008;134(5):677–699. [DOI] [PubMed] [Google Scholar]
- 60.Kushner HI.Why are there (almost) no left-handers in China? Endeavour. 2013;37(2):71–81. [DOI] [PubMed] [Google Scholar]
- 61.Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J.. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci U S A. 1997;94(25):14015–14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim SG, Ashe J, Hendrich K, et al. Functional magnetic resonance imaging of motor cortex: Hemispheric asymmetry and handedness. Science. 1993;261(5121):615–617. [DOI] [PubMed] [Google Scholar]
- 63.Angstmann S, Madsen KS, Skimminge A, Jernigan TL, Baaré WF, Siebner HR.. Microstructural asymmetry of the corticospinal tracts predicts right-left differences in circle drawing skill in right-handed adolescents. Brain Struct Funct. 2016;221(9):4475–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steinmetz H, Volkmann J, Jäncke L, Freund HJ.. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Ann Neurol. 1991;29(3):315–319. [DOI] [PubMed] [Google Scholar]
- 65.Galaburda AM, LeMay M, Kemper TL, Geschwind N.. Right-left asymmetrics in the brain. Science. 1978;199(4331):852–856. [DOI] [PubMed] [Google Scholar]
- 66.Wiberg A, Ng M, Al Omran Y, et al. Handedness, language areas and neuropsychiatric diseases: Insights from brain imaging and genetics. Brain. 2019;142(10):2938–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guadalupe T, Willems RM, Zwiers MP, et al. Differences in cerebral cortical anatomy of left- and right-handers. Front Psychol. 2014;5:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maingault S, Tzourio-Mazoyer N, Mazoyer B, Crivello F.. Regional correlations between cortical thickness and surface area asymmetries: A surface-based morphometry study of 250 adults. Neuropsychologia. 2016;93(Pt B):350–364. [DOI] [PubMed] [Google Scholar]
- 69.Hepper PG.The developmental origins of laterality: Fetal handedness. Dev Psychobiol. 2013;55(6):588–595. [DOI] [PubMed] [Google Scholar]
- 70.de Vries JI, Visser GH, Prechtl HF.. The emergence of fetal behaviour. II. Quantitative aspects. Early Hum Dev. 1985;12(2):99–120. [DOI] [PubMed] [Google Scholar]
- 71.Andescavage NN, Du Plessis A, McCarter R, et al. Complex trajectories of brain development in the healthy human fetus. Cereb Cortex. 2017;27(11):5274–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hering-Hanit R, Achiron R, Lipitz S, Achiron A.. Asymmetry of fetal cerebral hemispheres: In utero ultrasound study. Arch Dis Child Fetal Neonatal Ed. 2001;85(3):F194–F196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilmore JH, Lin W, Prastawa MW, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27(6):1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parma V, Brasselet R, Zoia S, Bulgheroni M, Castiello U.. The origin of human handedness and its role in pre-birth motor control. Sci Rep. 2017;7(1):16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandler WM, Paracchini S.. The genetic relationship between handedness and neurodevelopmental disorders. Trends Mol Med. 2014;20(2):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McManus IC, Martin N, Stubbings GF, Chung EM, Mitchison HM.. Handedness and situs inversus in primary ciliary dyskinesia. Proc Biol Sci. 2004;271(1557):2579–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vingerhoets G, Li X, Hou L, et al. Brain structural and functional asymmetry in human situs inversus totalis. Brain Struct Funct. 2018;223(4):1937–1952. [DOI] [PubMed] [Google Scholar]
- 78.Tanaka S, Kanzaki R, Yoshibayashi M, Kamiya T, Sugishita M.. Dichotic listening in patients with situs inversus: Brain asymmetry and situs asymmetry. Neuropsychologia. 1999;37(7):869–874. [DOI] [PubMed] [Google Scholar]
- 79.Cuellar-Partida G, Tung JY, Eriksson N, et al. Genome-wide association study identifies 48 common genetic variants associated with handedness. Nat Hum Behav. 2021;5(1):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Kovel CGF, Lisgo SN, Fisher SE, Francks C.. Subtle left-right asymmetry of gene expression profiles in embryonic and foetal human brains. Sci Rep. 2018;8(1):12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sha Z, Schijven D, Carrion-Castillo A, et al. The genetic architecture of structural left-right asymmetry of the human brain. Nat Hum Behav. 2021;doi: 10.1038/s41562-021-01069-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nikolova YS, Hariri AR.. Can we observe epigenetic effects on human brain function? Trends Cogn Sci. 2015;19(7):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Contestabile A, Sintoni S.. Histone acetylation in neurodevelopment. Curr Pharm Des. 2013;19(28):5043–5050. [DOI] [PubMed] [Google Scholar]
- 84.Rothbart SB, Strahl BD.. Interpreting the language of histone and DNA modifications. Biochim Biophys Acta. 2014;1839(8):627–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB.. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. [DOI] [PubMed] [Google Scholar]
- 86.Kilpinen H, Dermitzakis ET.. Genetic and epigenetic contribution to complex traits. Hum Mol Genet. 2012;21(R1):R24–R28. [DOI] [PubMed] [Google Scholar]
- 87.Jonsson H, Magnusdottir E, Eggertsson HP, et al. Differences between germline genomes of monozygotic twins. Nat Genet. 2021;53(1):27–34. [DOI] [PubMed] [Google Scholar]
- 88.de Kovel CGF, Carrión-Castillo A, Francks C.. A large-scale population study of early life factors influencing left-handedness. Sci Rep. 2019;9(1):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang Y, Chen S, Xu H, et al. Pre-gestational stress alters stress-response of pubertal offspring rat in sexually dimorphic and hemispherically asymmetric manner. BMC Neurosci. 2013;14:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaiserman AM.Epigenetic programming by early-life stress: Evidence from human populations. Dev Dyn. 2015;244(3):254–265. [DOI] [PubMed] [Google Scholar]
- 91.Turecki G, Meaney MJ.. Effects of the social environment and stress on glucocorticoid receptor gene methylation: A systematic review. Biol Psychiatry. 2016;79(2):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Letzner S, Patzke N, Verhaal J, Manns M.. Shaping a lateralized brain: Asymmetrical light experience modulates access to visual interhemispheric information in pigeons. Sci Rep. 2014;4:4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li P, Ensink E, Lang S, et al. Hemispheric asymmetry in the human brain and in Parkinson's disease is linked to divergent epigenetic patterns in neurons. Genome Biol. 2020;21(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiao FH, Wang HT, Kong QP.. Dynamic DNA methylation during aging: A “Prophet” of age-related outcomes. Front Genet. 2019;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karlebach G, Francks C.. Lateralization of gene expression in human language cortex. Cortex. 2015;67:30–36. [DOI] [PubMed] [Google Scholar]
- 96.Sun T, Patoine C, Abu-Khalil A, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308(5729):1794–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abu-Rustum RS, Ziade MF, Abu-Rustum SE.. Reference values for the right and left fetal choroid plexus at 11 to 13 weeks: An early sign of “developmental” laterality? J Ultrasound Med. 2013;32(9):1623–1629. [DOI] [PubMed] [Google Scholar]
- 98.Pletikos M, Sousa AM, Sedmak G, et al. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron. 2014;81(2):321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lambert N, Lambot MA, Bilheu A, et al. Genes expressed in specific areas of the human fetal cerebral cortex display distinct patterns of evolution. PLoS One. 2011;6(3):e17753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewek MD, Poole R, Johnson J, Halawa O, Huang X.. Arm swing magnitude and asymmetry during gait in the early stages of Parkinson's disease. Gait Posture. 2010;31(2):256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee CS, Schulzer M, Mak E, Hammerstad JP, Calne S, Calne DB.. Patterns of asymmetry do not change over the course of idiopathic parkinsonism: Implications for pathogenesis. Neurology. 1995;45(3 Pt 1):435–439. [DOI] [PubMed] [Google Scholar]
- 102.Kempster PA, Gibb WR, Stern GM, Lees AJ.. Asymmetry of substantia nigra neuronal loss in Parkinson's disease and its relevance to the mechanism of levodopa related motor fluctuations. J Neurol Neurosurg Psychiatry. 1989;52(1):72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E.. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 104.Uchihara T.An order in Lewy body disorders: Retrograde degeneration in hyperbranching axons as a fundamental structural template accounting for focal/multifocal Lewy body disease. Neuropathology. 2017;37(2):129–149. [DOI] [PubMed] [Google Scholar]
- 105.Tanner JJ, Levy SA, Schwab NA, et al. Marked brain asymmetry with intact cognitive functioning in idiopathic Parkinson's disease: A longitudinal analysis. Clin Neuropsychol. 2017;31(3):654–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ventura MI, Baynes K, Sigvardt KA, et al. Hemispheric asymmetries and prosodic emotion recognition deficits in Parkinson's disease. Neuropsychologia. 2012;50(8):1936–1945. [DOI] [PubMed] [Google Scholar]
- 107.Uitti RJ, Baba Y, Whaley NR, Wszolek ZK, Putzke JD.. Parkinson disease: Handedness predicts asymmetry. Neurology. 2005;64(11):1925–1930. [DOI] [PubMed] [Google Scholar]
- 108.Haaxma CA, Helmich RC, Borm GF, Kappelle AC, Horstink MW, Bloem BR.. Side of symptom onset affects motor dysfunction in Parkinson's disease. Neuroscience. 2010;170(4):1282–1285. [DOI] [PubMed] [Google Scholar]
- 109.Modestino EJ, Amenechi C, Reinhofer A, O'Toole P.. Side-of-onset of Parkinson's disease in relation to neuropsychological measures. Brain Behav. 2017;7(1):e00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedland RP, Koss E, Haxby JV, et al. NIH conference. Alzheimer disease: Clinical and biological heterogeneity. Ann Intern Med. 1988;109(4):298–311. [DOI] [PubMed] [Google Scholar]
- 111.Daianu M, Jahanshad N, Nir TM, et al. ; Alzheimer's Disease Neuroimaging Initiative. Breakdown of brain connectivity between normal aging and Alzheimer's disease: A structural k-core network analysis. Brain Connect. 2013;3(4):407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang C, Zhong S, Zhou X, Wei L, Wang L, Nie S.. The abnormality of topological asymmetry between hemispheric brain white matter networks in Alzheimer's disease and mild cognitive impairment. Front Aging Neurosci. 2017;9:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stefanits H, Budka H, Kovacs GG.. Asymmetry of neurodegenerative disease-related pathologies: A cautionary note. Acta Neuropathol. 2012;123(3):449–452. [DOI] [PubMed] [Google Scholar]
- 114.King A, Bodi I, Nolan M, Troakes C, Al-Sarraj S.. Assessment of the degree of asymmetry of pathological features in neurodegenerative diseases. What is the significance for brain banks? J Neural Transm (Vienna). 2015;122(10):1499–1508. [DOI] [PubMed] [Google Scholar]
- 115.Frings L, Hellwig S, Spehl TS, et al. Asymmetries of amyloid-β burden and neuronal dysfunction are positively correlated in Alzheimer's disease. Brain. 2015;138(10):3089–3099. [DOI] [PubMed] [Google Scholar]
- 116.Müller MJ, Greverus D, Dellani PR, et al. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage. 2005;28(4):1033–1042. [DOI] [PubMed] [Google Scholar]
- 117.Geroldi C, Akkawi NM, Galluzzi S, et al. Temporal lobe asymmetry in patients with Alzheimer's disease with delusions. J Neurol Neurosurg Psychiatry. 2000;69(2):187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Q, Mao C, Jin J, et al. Side of limb-onset predicts laterality of gray matter loss in amyotrophic lateral sclerosis. Biomed Res Int. 2014;2014:473250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blokhuis AM, Groen EJ, Koppers M, van den Berg LH, Pasterkamp RJ.. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125(6):777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Irwin DJ, McMillan CT, Xie SX, et al. Asymmetry of post-mortem neuropathology in behavioural-variant frontotemporal dementia. Brain. 2018;141(1):288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim G, Vahedi S, Gefen T, et al. Asymmetric TDP pathology in primary progressive aphasia with right hemisphere language dominance. Neurology. 2018;90(5):e396–e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim G, Ahmadian SS, Peterson M, et al. Asymmetric pathology in primary progressive aphasia with progranulin mutations and TDP inclusions. Neurology. 2016;86(7):627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG.. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. [DOI] [PubMed] [Google Scholar]
- 124.Pellegrino L, Coscia M, Muller M, Solaro C, Casadio M.. Evaluating upper limb impairments in multiple sclerosis by exposure to different mechanical environments. Sci Rep. 2018;8(1):2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larson RD, McCully KK, Larson DJ, Pryor WM, White LJ.. Bilateral differences in lower-limb performance in individuals with multiple sclerosis. J Rehabil Res Dev. 2013;50(2):215–222. [DOI] [PubMed] [Google Scholar]
- 126.Rudroff T, Kindred JH, Koo PJ, Karki R, Hebert JR.. Asymmetric glucose uptake in leg muscles of patients with Multiple Sclerosis during walking detected by [18F]-FDG PET/CT. NeuroRehabilitation. 2014;35(4):813–823. [DOI] [PubMed] [Google Scholar]
- 127.Filippi M, Preziosa P, Banwell BL, et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: Practical guidelines. Brain. 2019;142(7):1858–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prinster A, Quarantelli M, Orefice G, et al. Grey matter loss in relapsing-remitting multiple sclerosis: A voxel-based morphometry study. Neuroimage. 2006;29(3):859–867. [DOI] [PubMed] [Google Scholar]
- 129.Chaves AR, Wallack EM, Kelly LP, et al. Asymmetry of brain excitability: A new biomarker that predicts objective and subjective symptoms in multiple sclerosis. Behav Brain Res. 2019;359:281–291. [DOI] [PubMed] [Google Scholar]
- 130.Irwin P.Greater brain response of left-handers to drugs. Neuropsychologia. 1985;23(1):61–67. [DOI] [PubMed] [Google Scholar]
- 131.Barrett MJ, Wylie SA, Harrison MB, Wooten GF.. Handedness and motor symptom asymmetry in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2011;82(10):1122–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scherfler C, Seppi K, Mair KJ, et al. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson's disease. Brain. 2012;135(11):3348–3354. [DOI] [PubMed] [Google Scholar]
- 133.Yust-Katz S, Tesler D, Treves TA, Melamed E, Djaldetti R.. Handedness as a predictor of side of onset of Parkinson's disease. Parkinsonism Relat Disord. 2008;14(8):633–635. [DOI] [PubMed] [Google Scholar]
- 134.Turner MR, Wicks P, Brownstein CA, et al. Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(8):853–854. [DOI] [PubMed] [Google Scholar]
- 135.Devine MS, Kiernan MC, Heggie S, McCombe PA, Henderson RD.. Study of motor asymmetry in ALS indicates an effect of limb dominance on onset and spread of weakness, and an important role for upper motor neurons. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):481–487. [DOI] [PubMed] [Google Scholar]
- 136.Devine MS, Pannek K, Coulthard A, McCombe PA, Rose SE, Henderson RD.. Exposing asymmetric gray matter vulnerability in amyotrophic lateral sclerosis. Neuroimage Clin. 2015;7:782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gardener H, Munger K, Chitnis T, Spiegelman D, Ascherio A.. The relationship between handedness and risk of multiple sclerosis. Mult Scler. 2009;15(5):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shirani A, Cross AH, Naismith RT; Multiple Sclerosis Partners Advancing Technology and Health Solutions Investigators#. The association between handedness and clinicodemographic characteristics in people with multiple sclerosis: A brief report. Mult Scler J Exp Transl Clin. 2019;5(1):2055217319832031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Desikan RS, Schork AJ, Wang Y, et al. ; ADNI, ADGC, GERAD, CHARGE and IPDGC Investigators. Genetic overlap between Alzheimer's disease and Parkinson's disease at the MAPT locus. Mol Psychiatry. 2015;20(12):1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kapitein LC, Hoogenraad CC.. Building the neuronal microtubule cytoskeleton. Neuron. 2015;87(3):492–506. [DOI] [PubMed] [Google Scholar]
- 141.Millecamps S, Julien JP.. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14(3):161–176. [DOI] [PubMed] [Google Scholar]
- 142.Sorbara CD, Wagner NE, Ladwig A, et al. Pervasive axonal transport deficits in multiple sclerosis models. Neuron. 2014;84(6):1183–1190. [DOI] [PubMed] [Google Scholar]
- 143.Wachinger C, Nho K, Saykin AJ, Reuter M, Rieckmann A, Alzheimer’s Disease Neuroimaging Initiative. A longitudinal imaging genetics study of neuroanatomical asymmetry in Alzheimer's disease. Biol Psychiatry. 2018;84(7):522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Carrion-Castillo A, Pepe A, Kong XZ, et al. Genetic effects on planum temporale asymmetry and their limited relevance to neurodevelopmental disorders, intelligence or educational attainment. Cortex. 2020;124:137–153. [DOI] [PubMed] [Google Scholar]
- 145.Rothstein JD.Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65 (Suppl 1):S3–S9. [DOI] [PubMed] [Google Scholar]
- 146.Harwood CA, McDermott CJ, Shaw PJ.. Physical activity as an exogenous risk factor in motor neuron disease (MND): A review of the evidence. Amyotroph Lateral Scler. 2009;10(4):191–204. [DOI] [PubMed] [Google Scholar]
- 147.Dias V, Junn E, Mouradian MM.. The role of oxidative stress in Parkinson's disease. J Parkinsons Dis. 2013;3(4):461–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rocha EM, De Miranda B, Sanders LH.. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis. 2018;109(Pt B):249–257. [DOI] [PubMed] [Google Scholar]
- 149.Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P.. Triggers, facilitators, and aggravators: Redefining Parkinson's disease pathogenesis. Trends Neurosci. 2019;42(1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Strober BJ, Elorbany R, Rhodes K, et al. Dynamic genetic regulation of gene expression during cellular differentiation. Science. 2019;364(6447):1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pierce SE, Booms A, Prahl J, van der Schans EJC, Tyson T, Coetzee GA.. Post-GWAS knowledge gap: The how, where, and when. NPJ Parkinsons Dis. 2020;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Prasad S, Saini J, Yadav R, Pal PK.. Motor asymmetry and neuromelanin imaging: Concordance in Parkinson's disease. Parkinsonism Relat Disord. 2018;53:28–32. [DOI] [PubMed] [Google Scholar]
- 153.von Linstow CU, DeLano-Taylor M, Kordower JH, Brundin P.. Does developmental variability in the number of midbrain dopamine neurons affect individual risk for sporadic Parkinson's disease? J Parkinsons Dis. 2020;10(2):405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li Q, Bian S, Liu B, Hong J, Toth M, Sun T.. Establishing brain functional laterality in adult mice through unilateral gene manipulation in the embryonic cortex. Cell Res. 2013;23(9):1147–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tan YL, Yuan Y, Tian L.. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry. 2020;25(2):351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hickman S, Izzy S, Sen P, Morsett L, El Khoury J.. Microglia in neurodegeneration. Nat Neurosci. 2018;21(10):1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lun MP, Monuki ES, Lehtinen MK.. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci. 2015;16(8):445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Engelhardt B, Wolburg-Buchholz K, Wolburg H.. Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech. 2001;52(1):112–129. [DOI] [PubMed] [Google Scholar]
- 159.Sawamoto K, Wichterle H, Gonzalez-Perez O, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311(5761):629–632. [DOI] [PubMed] [Google Scholar]
- 160.Gross CC, Schulte-Mecklenbeck A, Hanning U, et al. Distinct pattern of lesion distribution in multiple sclerosis is associated with different circulating T-helper and helper-like innate lymphoid cell subsets. Mult Scler. 2017;23(7):1025–1030. [DOI] [PubMed] [Google Scholar]
- 161.Kutlu N, Mutlu F, Vural K, Cezayirli E.. Comparison of blood brain barrier permeability in normal and ovariectomized female rats that demonstrate right or left paw preference. Biotech Histochem. 2012;87(8):526–532. [DOI] [PubMed] [Google Scholar]
- 162.Rossor M, Garrett N, Iversen L.. No evidence for lateral asymmetry of neurotransmitters in post-mortem human brain. J Neurochem. 1980;35(3):743–745. [DOI] [PubMed] [Google Scholar]
- 163.Glick SD, Ross DA, Hough LB.. Lateral asymmetry of neurotransmitters in human brain. Brain Res. 1982;234(1):53–63. [DOI] [PubMed] [Google Scholar]
- 164.Molochnikov I, Cohen D.. Hemispheric differences in the mesostriatal dopaminergic system. Front Syst Neurosci. 2014;8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wagner HN Jr, Burns HD, Dannals RF, et al. Imaging dopamine receptors in the human brain by positron tomography. Science. 1983;221(4617):1264–1266. [DOI] [PubMed] [Google Scholar]
- 166.van Dyck CH, Seibyl JP, Malison RT, et al. Age-related decline in dopamine transporters: Analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry. 2002;10(1):36–43. [PubMed] [Google Scholar]
- 167.de la Fuente-Fernández R, Kishore A, Calne DB, Ruth TJ, Stoessl AJ.. Nigrostriatal dopamine system and motor lateralization. Behav Brain Res. 2000;112(1-2):63–68. [DOI] [PubMed] [Google Scholar]
- 168.Kesby JP, Eyles DW, McGrath JJ, Scott JG.. Dopamine, psychosis and schizophrenia: The widening gap between basic and clinical neuroscience. Transl Psychiatry. 2018;8(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brisch R, Saniotis A, Wolf R, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reynolds GP.Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature. 1983;305(5934):527–529. [DOI] [PubMed] [Google Scholar]
- 171.Reynolds GP, Czudek C.. Status of the dopaminergic system in post-mortem brain in schizophrenia. Psychopharmacol Bull. 1988;24(3):345–347. [PubMed] [Google Scholar]
- 172.Howes O, Bose S, Turkheimer F, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: A PET study. Mol Psychiatry. 2011;16(9):885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Markesbery WR, Jicha GA, Liu H, Schmitt FA.. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009;68(7):816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 175.Ma J, Gao J, Wang J, Xie A.. Prion-like mechanisms in Parkinson's disease. Front Neurosci. 2019;13:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goedert M, Masuda-Suzukake M, Falcon B.. Like prions: The propagation of aggregated tau and α-synuclein in neurodegeneration. Brain. 2017;140(2):266–278. [DOI] [PubMed] [Google Scholar]
- 177.Feiler MS, Strobel B, Freischmidt A, et al. TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol. 2015;211(4):897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Courte J, Bousset L, Boxberg YV, Villard C, Melki R, Peyrin JM.. The expression level of alpha-synuclein in different neuronal populations is the primary determinant of its prion-like seeding. Sci Rep. 2020;10(1):4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Henderson MX, Cornblath EJ, Darwich A, et al. Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat Neurosci. 2019;22(8):1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim S, Kwon S-H, Kam T-I, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron. 2019;103(4):627–641.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Freundt EC, Maynard N, Clancy EK, et al. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann Neurol. 2012;72(4):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW.. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73(6):1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Borghammer P, Van Den Berge N.. Brain-first versus gut-first Parkinson's disease: A hypothesis. J Parkinsons Dis. 2019;9(s2):S281–S295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–820. [DOI] [PubMed] [Google Scholar]
- 185.Ulusoy A, Rusconi R, Pérez-Revuelta BI, et al. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol Med. 2013;5(7):1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol. 2015;78(4):522–529. [DOI] [PubMed] [Google Scholar]
- 187.Killinger BA, Madaj Z, Sikora JW, et al. The vermiform appendix impacts the risk of developing Parkinson's disease. Sci Transl Med. 2018;10(465):eaar5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Römer C.Viruses and endogenous retroviruses as roots for neuroinflammation and neurodegenerative diseases. Front Neurosci. 2021;15(175):648629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fulop T, Witkowski JM, Bourgade K, et al. Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer's disease? Front Aging Neurosci. 2018;10:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Itzhaki RF, Lathe R, Balin BJ, et al. Microbes and Alzheimer's disease. J Alzheimers Dis. 2016;51(4):979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kumar DK, Choi SH, Washicosky KJ, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8(340):340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Limphaibool N, Iwanowski P, Holstad MJV, Kobylarek D, Kozubski W.. Infectious etiologies of parkinsonism: Pathomechanisms and clinical implications. Front Neurol. 2019;10:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Castanedo-Vazquez D, Bosque-Varela P, Sainz-Pelayo A, Riancho J.. Infectious agents and amyotrophic lateral sclerosis: Another piece of the puzzle of motor neuron degeneration. J Neurol. 2019;266(1):27–36. [DOI] [PubMed] [Google Scholar]
- 194.Xue YC, Feuer R, Cashman N, Luo H.. Enteroviral infection: The forgotten link to amyotrophic lateral sclerosis? Front Mol Neurosci. 2018;11:63–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Risberg J, Halsey JH, Wills EL, Wilson EM.. Hemispheric specialization in normal man studied by bilateral measurements of the regional cerebral blood flow. A study with the 133-Xe inhalation technique. Brain. 1975;98(3):511–524. [DOI] [PubMed] [Google Scholar]
- 196.Markus HS, Ring H, Kouris K, Costa DC.. Alterations in regional cerebral blood flow, with increased temporal interhemispheric asymmetries, in the normal elderly: An HMPAO SPECT study. Nucl Med Commun. 1993;14(8):628–633. [DOI] [PubMed] [Google Scholar]
- 197.Catafau AM, Lomeña FJ, Pavia J, et al. Regional cerebral blood flow pattern in normal young and aged volunteers: A 99mTc-HMPAO SPET study. Eur J Nucl Med. 1996;23(10):1329–1337. [DOI] [PubMed] [Google Scholar]
- 198.Van Laere K, Versijpt J, Audenaert K, et al. 99mTc-ECD brain perfusion SPET: Variability, asymmetry and effects of age and gender in healthy adults. Eur J Nucl Med. 2001;28(7):873–887. [DOI] [PubMed] [Google Scholar]
- 199.Cohen ME, Eichel R, Steiner-Birmanns B, et al. A case of probable Parkinson's disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19(10):804–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Faber I, Brandão PRP, Menegatti F, de Carvalho Bispo DD, Maluf FB, Cardoso F.. Coronavirus disease 2019 and parkinsonism: A non-post-encephalitic case. Mov Disord. 2020;35(10):1721–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Absinta M, Ha SK, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6:e29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ahn JH, Cho H, Kim JH, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572(7767):62–66. [DOI] [PubMed] [Google Scholar]
- 203.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560(7717):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ding XB, Wang XX, Xia DH, et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson's disease. Nat Med. 2021;27(3):411–418. [DOI] [PubMed] [Google Scholar]
- 206.Vandeputte C, Taymans JM, Casteels C, et al. Automated quantitative gait analysis in animal models of movement disorders. BMC Neurosci. 2010;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed.