5.
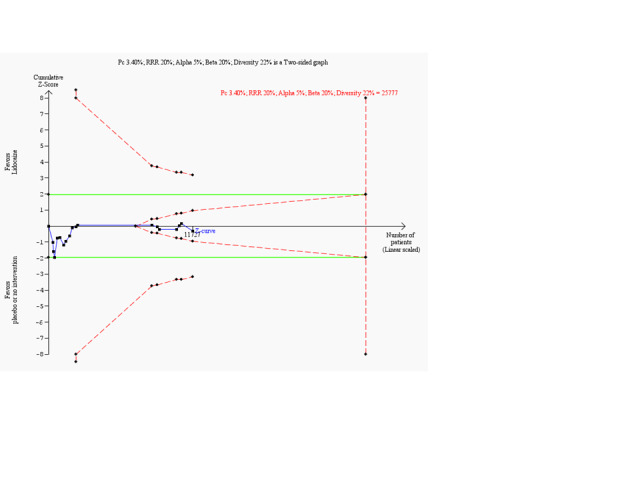
Trial sequential analysis on all‐cause mortality in 18 lidocaine vs placebo or no intervention trials
Trial sequential analysis of lidocaine vs placebo or no intervention on all‐cause mortality in participants with or without proven myocardial infarction based on the diversity‐adjusted required information size (DARIS) of 25,777 participants. This DARIS was calculated on the basis of a proportion of participants with suspected myocardial infarction of 3.40% in the control group; RRR of 20% in the experimental intervention group; alpha (α) of 5%; beta (β) of 20%; and diversity of 22%.
The cumulative Z‐curve (blue line) did not cross the conventional alpha 5% boundaries (green lines) at any time. After the 14th trial, the cumulative Z‐curve crosses the trial sequential monitoring boundary for futility. Accordingly, although only 45.5% (11,727/25,777) of the DARIS has been obtained, we can reject an intervention effect of 20% or larger. This implies that no additional trials may be needed to disprove an intervention effect of 20% relative risk reduction if bias can be ignored. Smaller risk reductions may require additional trials with larger sample sizes.
