10.
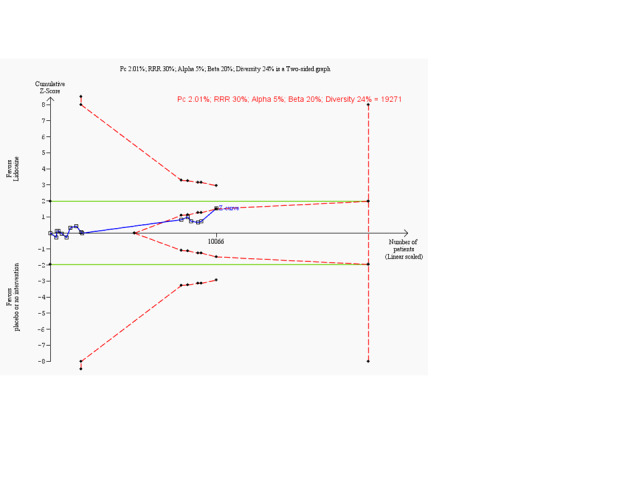
Trial sequential analysis on prevention of ventricular fibrillation in 15 lidocaine vs placebo or no intervention trials
Trial sequential analysis of lidocaine vs placebo or no intervention on prevention of ventricular fibrillation in participants with or without proven myocardial infarction based on the diversity‐adjusted required information size (DARIS) of 19,271 individuals. This DARIS was calculated on the basis of a proportion of participants with ventricular fibrillation of 2.01% in the control group; post hoc selected RRR of 30% in the experimental intervention group; alpha (α) of 5%; beta (β) of 20%; and diversity of 24%. The cumulative Z‐curve (blue line) did not cross conventional alpha 5% boundaries (green lines). After the 10th trial, the cumulative Z‐curve crosses the trial sequential monitoring boundary for futility. Accordingly, after only 52.2% (10,066/19,271) of the DARIS had been obtained, we were able to reject an intervention effect of 30% or larger. Had we calculated the DARIS on the basis of a more realistic RRR like 20% (as originally planned) or less, the obtained evidence would represent a smaller portion of the DARIS. Accordingly, the boundaries for futility would not have been crossed in such scenarios. Therefore, risk reductions of 20% or less may require additional trials with larger sample sizes.
